Once you found the object…. what would you do to avoid transferring the potential energy from the object and keep this bookshelf safe.

Answers
Answer:
put only the light items on it
Explanation:
Related Questions
A tablet weighing 0.940 g was dissolved in dilute sulphuric acid made up to 250 cm3 with water. 25.0 cm3 of this solution was titrated with 0.00160 M K2Cr2O7 requiring 32.5 cm3 of the K2Cr2O7. Calculate the percentage by mass of Fe2+ in the tablet.
Answers
A tablet weighing 0.940 g was dissolved in dilute sulphuric acid made up to 250 cm³ with water. 25.0 cm³ of this solution was titrated with 0.00160 M K\(_2\)Cr\(_2\)O requiring 32.5 cm3 of the K\(_2\)Cr\(_2\)O. 4.17% is the percentage by mass of \(Fe^{2+}\) in the tablet.
Mass percent is a means to describe a component in a specific combination or to convey a concentration. The mass percentage used to describe the solution composition indicates the mass of solute contained in a given mass of solution. The solute's concentration is specified in terms of mass or moles.
6\(Fe^2+\) + \(Cr_2O_7^{2-}\) + 14\(H^+\) → 6\(Fe^{3+}\) + 2\(Cr^{3+}\)+ 7H\(_2\)O
32.5 cm³x 0.00160 M = 0.052 g of K\(_2\)Cr\(_2\)O\(_7\)
0.052 g / 294.18 g/mol = 0.000177moles of K\(_2\)Cr\(_2\)O
0.000177 mol / 6 = 0.0000295 mol moles of \(Fe^{2+}\)
0.0000295 mol / 0.025 L = 0.00118 M of \(Fe^{2+}\)
0.00118 M x 55.845 g/mol x 0.025 L = 0.0392 g of \(Fe^{2+}\)
0.0392 g / 0.940 g x 100% = 4.17% percentage by mass of \(Fe^{2+}\)
To know more about mass percentage, here:
https://brainly.com/question/32197511
#SPJ12
Why must a solution of naoh be standardized using a primary standard, such as khp?.
Answers
NaOH is known to be adeliquescent substance, so it has to be standardized using KHP before used for analysis.
What is a primary standard?
A primary standard is a substance whose standard solution can be prepared using the solid. Such a substance must be pure and non-deliquescent.
Since NaOH is a deliquescent substance, it must therefore always be standardized using KHP before used for analysis.
Learn more about KHP: https://brainly.com/question/25893661
Need help im kinda stuck ;-;

Answers
Answer:
the answer is the third figure from left
A student prepares four different aqueous NaCl solutions according to the table. Which solution will have the highest molarity?

Answers
Brainly is erroneously blocking my answer from being posted due to nonexistent profanity and/or links, so I have attached an image of my written response. Please let me know if you have any problems seeing it.

If the mass is 12.3 g, volume without mineral is 50ml, volume with mineral is 53ml, then what is: (a) the volume of water displaced and (b) the final density of the mineral?
Answers
a.) The Volume of water displaced is 3 ml
b.) The final density of the mineral is 4.1 g/ml.
(a) The volume of water displaced is the ratio of the volume containing mineral to the volume excluding mineral.
Volume of water displaced = Volume with mineral - Volume without mineral
Volume of water displaced = 53 ml - 50 ml
Volume of water displaced = 3 ml.
(b) The following formula can be used to determine the mineral's density:
Mass / Volume equals density.
The difference between the mass of the mineral and the mass without the mineral is the mass of the mineral.
Mass of mineral = Mass with mineral - Mass without mineral
Mass of mineral = 12.3 g - 0 g (since the mass without mineral is not given)
Mass of mineral = 12.3 g
By deducting the volume without the mineral from the volume with, one may determine the volume of the mineral.
Volume of mineral = Volume with mineral - Volume without mineral
Volume of mineral = 53 ml - 50 ml
Volume of mineral = 3 ml
Therefore, the density of the mineral is:
Density = Mass of mineral / Volume of mineral
Density = 12.3 g / 3 ml
Density = 4.1 g/ml
Therefore, the final density of the mineral is 4.1 g/ml.
For such more question on Volume:
https://brainly.com/question/29796637
#SPJ11
If a sample of radioactive isotopes takes 60 minutes to decay from 200 grams to 50 grams, what is the half-life of the isotope
Answers
The radioactive atom in this sample has a half-life of about 138.6 minutes.
The half-life of a radioactive isotope is the time required for half of the atoms in a sample to decay. The half-life of an isotope depends on its specific decay rate, which is determined by its nuclear properties.
In this case, the sample of radioactive isotopes decays from 200 grams to 50 grams over a period of 60 minutes. We can use this information to calculate the half-life of the isotope using the following equation:
N = N₀ x \((1/2)^(t/T)\)
where N is the final amount of the isotope (50 grams), N₀ is the initial amount of the isotope (200 grams), t is the time elapsed (60 minutes), and T is the half-life of the isotope (in minutes).
Substituting the given values into the equation, we get:
50 = 200 x \(1/2^{(60/T)}\)
Dividing both sides by 200 and taking the natural logarithm of both sides, we get:
ln(1/4) = -60/T
Solving for T, we get:
T = -60 / ln(1/4) ≈ 138.6 minutes
Therefore, the half-life of the radioactive isotope in this sample is approximately 138.6 minutes.
To learn more about isotopes refer to:
brainly.com/question/5357969
#SPJ4
The energy required to ionize boron is 801 kJ/mol. What minimum frequency of light is required to ionize boron?
Answers
The minimum frequency of light required to ionize boron is approximately 2.01 x 10^15 Hz.
To find the minimum frequency of light required to ionize boron, we can use the equation E = hf, where E is the energy required to ionize boron and f is the frequency of light.
Given that the energy required to ionize boron is 801 kJ/mol, we can convert this to joules by multiplying by the conversion factor 1000 J/1 kJ and dividing by Avogadro's number (6.022 x 10^23 mol^-1) to get the energy per atom.
801 kJ/mol * (1000 J/1 kJ) / (6.022 x 10^23 mol^-1) = 1.33 x 10^-18 J
Now, we can rearrange the equation to solve for the frequency of light:
f = E / h
Substituting the values:
f = (1.33 x 10^-18 J) / (6.626 x 10^-34 J·s) = 2.01 x 10^15 Hz
Therefore, the minimum frequency of light required to ionize boron is approximately 2.01 x 10^15 Hz.
Learn more:About ionization here:
https://brainly.com/question/1602374
#SPJ11
Ionization energy is the energy required to remove an electron from an atom or ion in its ground state. It is often expressed in units of joules (J) or kilojoules per mole (kJ/mol). 2.01 x 10¹⁵ Hz is the minimum frequency of light is required to ionize boron
Ionization energy per atom
= ionization energy per mole/Avogadro's number
= 801/6.022 x 10²³
= 1.330 x 10⁻²¹ kJ = 1.330 x 10⁻¹⁸ J
Photon energy E = hf
where h is the Planck constant and f is the frequency
Photon energy = ionization energy per atom
E=hf = 1.330 x 10⁻¹⁸
Frequency f = 1.330 x 10⁻¹⁸/6.626 x 10⁻³⁴
= 2.01 x 10¹⁵ Hz
To know more about Ionization energy:
https://brainly.com/question/28385102
#SPJ4
4) How many grams are in 4.63 x 102 moles of CC14?
Answers
Answer:
n=mass/molar mass
mass=?,molar mass=12+(35.5)4=154g/mol.n=4.63x10²
mass=4.63x10²x154=7128g
How will increased physical activity affect heart rate?
Answers
Answer:
when we do increased activity our body requires more energy and therefore more oxygen and
therefore heart has to pump blood much faster and that's why increase heart rate
Answer:
do some jumping activity and other exercises
Explanation:
hope it helps
A solution contains 20.1 grams of CaCO3 in 2.0 L of water.
Answers
According to molar concentration, the molarity of a solution containing 20.1 grams of CaCO₃ in 2.0 L of water is 0.1004 M.
Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molarity=mass/ molar mass ×1/volume of solution in liters.Substitution of values in formula gives molarity= 20.1/100.08×1/2=0.1004 M.
Thus, the molarity of a solution containing 20.1 grams of CaCO₃ in 2.0 L of water is 0.1004 M.
Learn more about molar concentration ,here:
https://brainly.com/question/21841645
#SPJ1
Your question is incomplete,but most probably your full question was, a solution contains 20.1 grams of CaCO₃ in 2.0 L of water.What is it's molarity?
According to the bohr model of the atom, the single electron of a hydrogen atom circles the nucleus...
a. in specific, allowed orbits.
b. in one fixed orbit at all times.
c. at any of an infinite number of distances, depending on its energy.
d. counterclockwise.
Answers
The protons and neutrons that make up the atom's nucleus account for the majority of the atom's mass according to the Bohr model.
What is meant by bohr model of the atom?The Bohr model, also known as the Rutherford-Bohr model, was first proposed by Niels Bohr and Ernest Rutherford in 1913. It is a system made up of an orbiting nucleus that is compact and dense, surrounded by electrons, much like the Solar System.
Bohr was the first to realise that electrons move in various orbits around the nucleus and that the quantity of electrons in an element's outer orbit determines its properties.
In the Bohr model of the atom, the protons and neutrons that make up the nucleus are responsible for most of the atom's mass. The negatively charged electrons orbit the positively charged core, making up a small portion of the mass but being electrically comparable to the protons in the nucleus.
Therefore, the correct answer is option a. in specific, allowed orbits.
To learn more about bohr model refer to;
https://brainly.com/question/4138548
#SPJ4
Someone help me quick! Will give brainliest
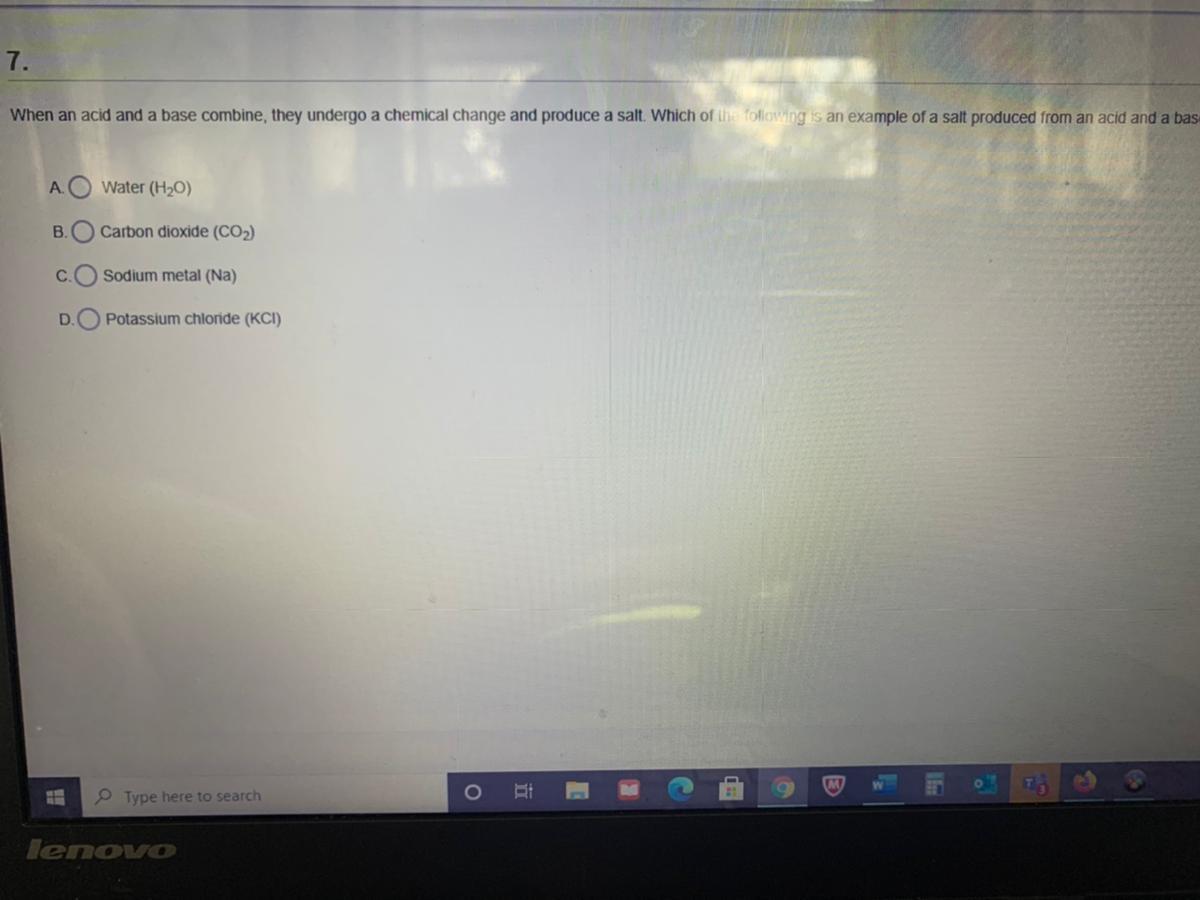
Answers
Answer:
WHY DO YOU NEED HELP HUH
DON'T CHEAT READ AND STUDY
Explanation:
Answer:
It's C
Explanation:
At 1.3 atm and 23 degrees Celsius, 12.5 grams of a gas occupies a volume of 8.2 liters. What is the molar mass (grams/mole) of the gas?
Answers
Answer:
0.44 moles Molar Mass = 28.496
Explanation:
1.3(8.2)=n(0.0821)(23+273)
PV=nRT
12.5/0.43865
A molecular compound is formed when a chemical reaction occurs between atoms of * 4 points Strontium and chlorine Magnesium and fluorine Sulfur and Nitrogen Oxygen and calcium
Answers
Answer:
Sulfur and Nitrogen
Explanation:
Molecular compounds are formed mostly when nonmetals combine. Usually electrons are shared between atoms in the covalent bond.
Sulphur and nitrogen are nonmetals whose difference in electronegativity is not much. Hence they form a molecular compound.
Where is an electron located within an atom?
O A. Anywhere within the atom
B. Attached to a proton
C. In the nucleus of the atom
D. In the cloud around the nucleus
Answers
Answer:
D because electron is negatively charge around the nucleus
electrons are found outside the nucleus
An atoms is composed of electrons, protons and neutrons. Protons and neutrons are attached within the nucleus whereas electrons are in the cloud around the nucleus. Thus, option D is correct.
What is an atom?Atoms are the basic units of every substance. Atoms are composed of subatomic particles electrons, neutrons and protons. Protons and neutrons are located inside the nucleus where as electrons are revolving around the nucleus.
The core of an atom is called nucleus. The protons are positively charged and the neutrons are neutral. Electrons are negatively charged particles and they are revolving around the nucleus through circular paths of fixed energy called orbits.
The region where we can find an electron inside an atom is called an orbital. Electrons experience an attractive pull from the positively charged nucleus and thus electrons are existing in a cloud of electrostatic force around nucleus.
To find more on electrons, refer here:
https://brainly.com/question/1255220
#SPJ5
If 10 grams of potassium chlorate decompose to form potassium chloride and oxygen gas inside a 500 mL container at a temperature of 45 °C. what will the pressure be inside the container?
Answers
Answer:
10 atm
Explanation:
There's a lot to do here, but lets take it one step at a time. First, let's write a balanced equation for the decomposition of potassium chlorate into potassium chloride and oxgyen gas.
2 KClO3 → 2 KCl + 3 O2
Now let's find the moles of the KClO3 (molar mass 122.55 g/mol) that we have take 10 g/122.55 g/mol, grams will cancel and we are left with 0.0816 moles. lets divide that by two since we have a two in front of the KClO3 in the equation, and then multiply that number by 5 since it's the total moles of products, in summary, multiply by 5/2 to get 0.204 moles.
Now that we know the moles of our products, let's plug some stuff into the ideal gas law PV = nRT. We are looking for P so let's solve for that. P = (nRT)/V, now let's plug in our values. Make sure V is converted to liters so 0.5 L. And convert celcius to kelvin by adding 273
P = ((0.204 moles)(318 K)(0.08206 L atm mol^-1 K^-1))/0.5 L
A lot of units cancel, and we get about 10.65 atm, if you don't want the answer in atm, you can find a conversion equation. But let's round to sig figs for now, which will bring us to 10 atm.
Path 2 is a straight line because of the alpha particles'
O magnetic repulsion
O high velocity
O distance from gold nuclei
O interaction with electrons

Answers
Answer:
C distance from gold nuclei
HELP ASAP!!!
Which of the following is a function of the cell membrane?
Producing energy
Creating new cells
Eliminating of waste
Replacing damaged cells
Answers
It took 3 hours for a train to travel the distance between two cities at a
velocity of 290 miles/hour. What is the DISTANCE between the two cities?
Pls hurry!
Answers
Answer:
The answer is 870 milesExplanation:
The distance covered by an object given it's velocity and time taken can be found by using the formula
distance = velocity × timeFrom the question
velocity = 290 miles/hour
time = 3 hours
The distance between the two cities is
distance = 290 × 3
We have the final answer as
870 milesHope this helps you
Which might cause someone to be exempt from soccer practice? *
a. an injury
b. a previous absence
Answers
Answer:
injury
Explanation:
because get hurt we can say injury
Free nerve endings in the skin detect changes in skin temperature (getting warmer).
Answers
how many calories are in a dinner containing 3.5x10 ^6 joules
Answers
Answer:
3500000 try this i think it's right not sure
Explanation:
Calories are used to define the unit of energy given by the consumed food in the body. A dinner containing 3.5x10⁶ joules has calories of 836.52 Cal.
What are calories?Calories have been defined as the amount of energy given by the food and its various components, including carbohydrates, proteins, fats, fibers, etc. The stored calories more than the use of those calories results in their storage in the adipose tissues which may lead to obesity.
The food energy from joules can be converted into large calories (Cal) as it is the required amount to raise 1°C of the temperature of a 1 kg substance at 1 atm pressure.
The conversion is done as:
1 J = 0.000239 Cal
3500000 joules = 836.520 Cal
Therefore, the energy of dinner in calories is 836.520 Cal.
Learn more about calories, here:
https://brainly.com/question/22374134
#SPJ2
the transfer of thermal energy that causes a metal spoon in hot liquid to get hot.
Answers
Answer:
Conduction
Explanation:
Conduction can be regarded as transfer of thermal energy which exist
between two or more objects that are in contact with each other. From the question, the spoon touches the hot liquid and heat is transferred to the spoon through conduction
Wk-14
Date
Page
Assignment
1. Calculate the percentage of iron in K3 Fe (CN)
[K= 39, Fe =56, C= 12, N =14]
Answers
Answer:
The mass percentage of iron in \(\rm K_3[Fe(CN)_6]\) (potassium ferricyanide) is approximately \(17\%\).
Explanation:
Start by finding the formula mass of potassium ferricyanide, \(\rm K_3[Fe(CN)_6]\), using relative atomic mass data from the question:
\(\begin{aligned} & M(\mathrm{K_3[Fe(CN)_6]}) \\ &\approx 3 \times 39 + 56 + 6 \times (12 + 14) \\ &= \rm 329 \; g \cdot mol^{-1}\end{aligned}\).
In other words, every one mole of \(\rm K_3[Fe(CN)_6]\) formula units have a mass of approximately \(329\; \rm g\).
On the other hand, note that there is one iron \(\rm Fe\) atom in every one mole of \(\rm K_3[Fe(CN)_6]\) formula units. Hence, there would be exactly one mole of iron atoms in every one mole of \(\rm K_3[Fe(CN)_6]\) formula units. The mass of that one mole of iron atoms is approximately \(56\; \rm g\) (again, from the relative atomic mass data of the question.)
Therefore:
\(\begin{aligned}& \text{Mass percentage of $\mathrm{Fe}$ in $\mathrm{K_3[Fe(CN)_6]}$} \\ &= \frac{\text{Mass of $\mathrm{Fe}$ in one mole of $\mathrm{K_3[Fe(CN)_6]}$ formula units}}{\text{Mass of one mole of $\mathrm{K_3[Fe(CN)_6]}$ formula units}}\times 100\% \\ &= \frac{M(\mathrm{Fe}) \times (\text{Number of $\mathrm{Fe}$ atoms in each $\mathrm{K_3[Fe(CN)_6]}$ formula unit)}}{M(\mathrm{K_3[Fe(CN)_6]})} \times 100\%\end{aligned}\)
\(\begin{aligned}&\approx \frac{1 \times 56\; \rm g \cdot mol^{-1}}{329\; \rm g \cdot mol^{-1}}\end{aligned}\).
help meeeee
I require someone WITH braincells
22 How does Earth's tilt affect climate?
OA. smaller the tilt, the warmer the summer.
OB. The smaller the tilt, the smaller the temperature difference is between summer and winter.
OC. The larger the tilt, the warmer the summer.
OD. The larger the tilt, the smaller the temperature difference is between summer and winter.
Answers
which of the following is not a chemical property of matter

Answers
the answer is letter "d"
Explanation:
bcoz the change of shape doesn't involve chemical reactions...
d
dont have to explain
ok mark pls pls pls pls pls
Explain why you need to use a clean pipette tip to transfer each DNA sample to the gel plate.
Answers
Answer: Sterilization
Explanation:
The pipette needs to be sterile so that nothing interferes with the DNA sample.
please help due todays (show your to)!!!

Answers
Answer:28.411
Explanation: f1 m1+ f2 m2+ f3 m3
0.6628(28)+0.2634(29)+0.0738(30)=28.411
remember that for percentages you divide the number by 100 to get the decimal form.
A first-order reaction that results in the destruction of a pollutant has a rate constant of 0.1/day. how many days will it take for 68 percent of the chemical to be destroyed?
Answers
In a first-order reaction, it takes 23.02 days for 68 percent of the chemical to be destroyed.
Given,
fee constant for the destruction of pollutant = 0.1/day
We ought to discover the number of days it'll take for sixty eight percent of the chemical to be destroyed.
[A] = [A]₀e^⁻kt
in which [A] is the present awareness, and
[A]₀ is the initial awareness
k is the price of decrement
[A]₀ = 1
[A] = 0.1
Or, t =㏑(10)/k
Or, t = 2.302/zero.1
Or, t = 23.02 days.
for this reason the wide variety of days required for the destruction of sixty eight percent of chemicals is 23 days.
To learn more about the first-order reaction, visit: https://brainly.com/question/10898529
#SPJ4
Question 5 How many grams of Hydrogen will be created from 5 moles of Aluminum? 2 Al + 3 H2SO4 – Al2(SO4)3 + 3H2
Answers
Answer:
Solution given:
2 Al + 3 H2SO4 – Al2(SO4)3 + 3H2
2mole +3mole. --- 1 mole + 3 mole
27×2g. +3×98g-------- 342,g. + 6g
we have
2 mole of Al gives. 6g of h2
5 moles of Al gives 6/2×5=15g of hydrogen
15 g is your answer