Answers
Answer:
The valence is two.
Explanation:
The valency is two because that is how many electrons it exchanges with another atom we it comes in contact.
Answer:
The valence is two.
Explanation:
The valency is two because that is how many electrons it exchanges with another atom we it comes in contact.
Related Questions
How many grams are in 1.25 moles of NaCl
Answers
Answer:
73.05g
Explanation:
1.25 mol NaCl * \(\frac{58.44g}{1 mol NaCl}\) = 73.05g
58.44g = molar mass of NaCl
Which of the following compounds would most likely be the reactants in a combustion reaction?
sulfuric acid and water
oxygen and hydrocarbon
oxygen and carbon dioxide
carbon dioxide and hydrocarbon
Answers
Answer:
reaction between oxygen and hydrocarbon is combustion
The compound would most likely be the reactants in a combustion reaction is oxygen and hydrocarbon.
What is combustion reaction?Those reaction in which a fuel will change into a water molecule and carbon dioxide gas, in the presence of oxygen is known as combustion reaction.
Combustion reaction will represent as:
CₓHₙ + O₂ → H₂O + CO₂, where
CₓHₙ is a hydrocarbon which gets oxidized in the presence of oxygen oxidant.
Hence option (2) is correct i.e. oxygen and hydrocarbon.
To know more about combustion reaction, visit the below link:
https://brainly.com/question/9425444
When 0.500 g of Ca was burned in oxygen in a constant volume calorimeter, 7.92 kJ of energy as heat was evolved. The calorimeter was in an insulated container with 720. g of water at an initial temperature of 19.2 °C. The heat capacity of the bomb in the calorimeter is 600. J/K. The specific heat capacity of water is 4.184 J/g ⋅ °C. Calculate △U for the oxidation of Ca (in kJ/mol Ca). △U = ____ kJ/mol Ca
Answers
The ΔU for the oxidation of Ca is 634.176 kJ/mol Ca.
To calculate ΔU for the oxidation of Ca, we need to consider the energy transferred as heat in the reaction and the molar amount of Ca involved.
First, let's determine the amount of heat transferred during the reaction. We are given that 7.92 kJ of energy as heat was evolved. Since the reaction took place in a constant volume calorimeter, this heat transferred is equal to the change in internal energy (ΔU) of the system.
Next, we need to calculate the mass of Ca used in the reaction. We are given that 0.500 g of Ca was burned.
To calculate ΔU in kJ/mol Ca, we need to convert the mass of Ca to moles. The molar mass of Ca is 40.08 g/mol.
Now, let's calculate the moles of Ca:
moles of Ca = mass of Ca / molar mass of Ca
moles of Ca = 0.500 g / 40.08 g/mol
Now that we have the moles of Ca, we can calculate ΔU in kJ/mol Ca:
ΔU = heat transferred / moles of Ca
ΔU = 7.92 kJ / (0.500 g / 40.08 g/mol)
Simplifying the expression:
ΔU = 7.92 kJ * (40.08 g/mol) / 0.500 g
Calculating ΔU:
ΔU = 634.176 kJ/mol Ca
Therefore, the ΔU for the oxidation of Ca is 634.176 kJ/mol Ca.
Please note that the unit for ΔU is kJ/mol Ca, indicating the change in internal energy per mole of Ca involved in the reaction.
For more such question on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
Are the gas laws affected depending on whether you use heavy gas particles or light gas particles? If anything, what is affected by the size of the molecule?
Answers
Answer:
yes
Explanation:
Yes, size of molecule has greatly affected the gas law.
The gas laws affected depending on whether we use heavy gas particles or light gas particles because the heavy gas particles can't move faster as light gas particles with the increase of temperature.
If the temperature is increased, the gas particles move faster due to absorb of heat energy so they hit the walls of the container. This causes the pressure to rise. The pressure of a gas also increases when the volume of container in which it is placed is decreased. Bigger the molecule size, the stronger the intermolecular forces, so the molecule has higher melting and boiling points so we can conclude that big size molecule greatly affected the gas law.
Learn more: https://brainly.com/question/21635224
What is the molecular weight of MgCl2
Answers
The molecular weight of MgCl₂ is 94 g/mol.
What is molecular weight?This refers to the total mass of a compound. It is equivalent to the sum of the individual atomic masses of each atom in the molecule.
Steps to determine the molecular formula of the molecule.
Determine the atomic mass of each element in the molecule.Multiply each element's atomic mass by the number of atoms of that element in the molecule. This number is represented by the subscript next to the element symbol in the molecular formula.Add these values together for each different atom in the molecule.The total will be the molecular mass of the compound.Atomic number of Cl =17
Atomic number of Mg = 12
Mass number of Cl = 35
Mass number of Mg = 24
Calculating the molecular weight of MgCl₂
MgCl₂
=(12* 2) + (35*2)
= 24 + 70
= 94 g/mol.
Learn more about molecular weight on
https://brainly.com/question/13146190
#SPJ1
4.939 g smaple of sodium nitrite is dissolved in 50.084 g of dionized water
Answers
The heat of solution of sodium nitrite is 17672.32 J.
What is the heat of solution of the sodium nitrite?The heat of solution of sodium nitrite is calculated from the formula given as follows:
Heat change, Δq = mcΔT
where;
m is massc is the specific heat capacityΔT is the temperature changem = 4.939 + 50.084
m = 55.023
c = 4.18 J/g/°C
ΔT = (20 - 14.5)
ΔT = 5.5 °C
Δq = 55.023 * 4.18 * 5.5
Δq = 1264.98 J
Heat of solution = Δq / moles of sodium nitrite
Moles of sodium nitrite = mass / molar mass
molar mass of sodium nitrite = 69 g/mol
Heat of solution = 1264.98 J / (4.939/69) mol
Heat of solution = 17672.32 J
Learn more about heat of solution at: https://brainly.com/question/28169738
#SPJ1
Complete question:
A 4.939 g sample of sodium nitrate is dissolved in 50.084 g of distilled water, initially at 20.0 °C. The temperature of the water fell to 14.5 °C after the sample dissolved. Find
a. the heat of solution in joules
a student performs a gravimetric analysis experiment and is given 1.94 g 1.94 g of a contaminated mixture containing anhydrous magnesium chloride and potassium nitrate. to determine the percentage by mass of magnesium chloride in the mixture, excess silver nitrate is added to the mixture to precipitate the chloride ion as silver chloride. the mass of the silver chloride precipitate is found to be 1.43 g 1.43 g. which of the following is the mass percent of magnesium chloride in this sample?
a.25%
b.24%
c. 47%
d. 74%
Answers
The only material that will precipitate is silver nitrate. Silver chloride will precipitate as a result of the reaction between magnesium chloride and silver nitrate. 1.94 g 1.94 g of a contaminated combination including anhydrous potassium magnesium chloride and magnesium chloride is supplied to the subject of a gravimetric analysis experiment.
What is anhydrous magnesium?
The student uses extra AgNO3(aq) to precipitate the chloride ion as AgCl to determine the mixture's proportion by mass of MgCl2 (s). To ascertain the sodium chloride content, a gravimetric study is conducted. The ratio of magnesium chloride to silver nitrate is 1:2, meaning that a mineral water should use a minimum of two moles of silver nitrate while precipitating the chloride ions as silver chloride. Using gravimetric analysis, a student wants to ascertain the percentage of mass.
To learn more about potassium nitrate from given link
brainly.com/question/15080595
#SPJ4
Need it in 2 minutes ASAP


Answers
Answer: read your book
Explanation: if you read it should be in there
What does this image represent?
Amine group
Carbonyl group
Ether group
Hydroxyl group
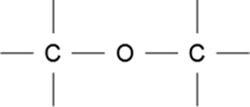
Answers

Answer:
ether group
Explanation: I looked it up
The molecule β-carotene has λ 450 nm, and ɛ = 15,000 m2 mol-1. Calculate the absorption (A) expected for a solution in which 0.1 mg has been dissolved in 10 ml of water (given: the molecular weight of β-carotene, C40H56, as 536) with a path length of 1 cm. Group of answer choices
Answers
Answer: The absorbance for a solution is 0.0028
Explanation:
To calculate the absorption of a solution, the equation by Beer-Lambert law is used:
\(A=\varepsilon \times b\times C\)
OR
\(A=\varepsilon \times b\times \frac{\text{Given mass of solute}\times 1000}{\text{Molar mass of solute}\times \text{Volume of solution (mL)}}\)
where,
A = absorbance = ?
\(\varepsilon\) = molar absorptivity = \)15000m^2mol^{-1}L\)
b = path length = 1 cm = 0.01 m (Conversion factor: 1 m = 100 cm)
Given mass of \(\beta-\) carotene = 0.1 mg = 0.0001 g (Conversion factor: 1 g = 1000 mg)
Molar mass of \(\beta-\) carotene = 536 g/mol
Volume of solution = 10 mL
Putting values in equation 1:
\(A=15000\times 0.01\times \frac{0.0001\times 1000}{536\times 10}\\\\A=0.0028\)
Hence, the absorbance for a solution is 0.0028
How does a plant get and use energy?
Drag and drop the steps of the process to show the correct order.

Answers
The steps of how plants get energy in the correct order is as follows:
Sunlight shines on the leaves of a plant (option B). Cells in the leaves perform photosynthesis (option A). Glucose and other sugars travel throughout the plant (option D). Plant cells break apart the sugars to release their energy (option C) What is photosynthesis?Photosynthesis is the process by which plants and other photoautotrophs convert light energy into chemical energy.
Photosynthesis is carried out by the cells of green plants to synthesize their food in form of sugar powered by the energy from sunlight.
The cells in the leaf use the energy from the sun (light energy) and produce sugars (chemical energy). After which, the sugars are broken down to release energy for use by the cells in a process called cellular respiration.
Learn more about photosynthesis at: https://brainly.com/question/29764662
#SPJ1
Which of the following metals has a low melting point?
2 A. Rubidium
B. Potassium
C. Calcium
D. Sodium
Answers
Answer:
Rubidium
Explanation:
Choose the correct option.
1. A chain of small chemical units combined to form a large single unit is called ______
a) Polymer
b) Poly
c) Polythene
d) None of the above
2. Polythene and PVC are examples of
a) Bio degradable substance
b) Thermosetting plastics
c) Thermoplastics
d) Rayon
3. Plastics which when moulded once, cannot be softened by heating. Such plastics are called __ a) Polythene
b) Thermoplastics
c) Polyster
d) Thermosetting plastics
4. Polycot is made by mixing two types of fibres namely
a) Silk + Cotton
b) Polythene + cotton
c) Silk + Polyester
d) Polyester + Cotton
5. The 4 R Principle is
a) Reduce, Reuse, Recycle, Recover
b) Remember, reduce, Recycle, Rejoice
c) Repeat, Rejoice, recycle, reduce
d) None of the above
6. _____________ is an example of natural polymer
a) Rayon
b) Cellulose
c) Nylon
d) All of the above
7. Which of the following is Non-biodegradable ?
a) Woolen clothes
b) Plastic bag,
c) Cotton cloth
d) Wood
8. Bakelite and Melamine are examples of
a) Thermosetting plastics
b) Silk
c) Nylon
d) Rayon
9. Fire proof plastic uniform worn by fire fighters has a coating of _____ to make it fire resistant. a) Nylon
b) Rayon
c) Melamine plastic
d) silk
The coating on modern non- stick cookware and electric iron is of
a) Terrycot
b) Rayon
c) Polyester
d) Teflon
Answers
2 Thermoplastic
3 Thermosetting plastics
4 polyester + cotton
5 Reduce, reuse, recycle, recover
6. Cellulose
7 wood
8 thermosetting plastic
9silk
10 Teflon
I understand how a change in the size of the moon jellies' resource population can change the number of births in the moon jelly population.
Responses
Explain your answer choice.
Answers
A change in the size of the moon jellies' resource population can change the number of births in the moon jelly population because the big size of the resources can produce more births.
How do moon jellies reproduce?When there is more energy storage molecules present in the moon jellies, they can reproduce more, in more births. Fewer deaths would also lead to the jelly population increasing. The sea turtle population, and the moon jellies consumer population is also decreased.
There must be a change to the birth rate or the death rate in the moon jelly population. Within a population, organisms are born and dying continuously. If the number of births and deaths in a given time interval are equal, then the population size will remain stable.
So we can conclude that a large population of resources will lead to more births.
Learn more about jellies here: https://brainly.com/question/25630111
#SPJ1
Please help! Thanks in advance.

Answers
Answer:
question 1, i'd say not enough information
question 2, do you know of potassium bromine lowers the entropy of the water solution or raises the entropy level? if so entropy is either A or C
question 3, i'd say entropy is less than 0, but i don't know because i never learned this in school
Explanation:
we don't know the original volume of gas and only know the elements that are in it
A sample of gas has a volume of 100. L at 17 °C and 800. torr. To what temperature must the gas be cooled in order for its volume to become 50.0 L at a pressure of 600. torr? Your answer will need to be in Kelvin.
Source
StylesNormalFontSize
Answers
Answer:
108.81 K
Explanation:
First convert 17 °C to Kelvin:
17 + 273.16 = 290.16 KAssuming ideal behaviour, we can solve this problem by using the combined gas law, which states that at constant composition:
P₁V₁T₂=P₂V₂T₁Where in this case:
P₁ = 800 torrV₁ = 100 LT₂ = ?P₂ = 600 torrV₂ = 50 LT₁ = 290.16 KWe input the data:
800 torr * 100 L * T₂ = 600 torr * 50 L * 290.16 KAnd solve for T₂:
T₂ = 108.81 Kscientific report on esterificatin
Answers
Esterification is a chemical reaction between an alcohol and a carboxylic acid that results in the formation of an ester and a molecule of water.
Write a scientific report on esterificationEsterification is an important class of organic reactions and is widely used in the synthesis of flavors, fragrances, and plastics. In this report, we will explore the fundamental principles and practical applications of esterification.
The experimental procedure involves the reaction between methanol and acetic acid to form methyl acetate and water. The reaction was carried out in a round-bottom flask equipped with a condenser and a thermometer. The reactants were mixed in stoichiometric amounts, and a small amount of sulfuric acid was added as a catalyst. The flask was heated using a hot plate and maintained at a constant temperature of 60°C.
Learn more about esterification:https://brainly.com/question/16010744
#SPJ1
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
How many moles of water are needed to react to produce 5.0 moles of sodium hydroxide?
Please help!!!

Answers
Answer:
2,5
Explanation:
How many milliliters of an aqueous solution of 0.124 M silver fluoride is needed to obtain 17.8 grams of the salt? mL
Answers
We need 117.76 mL volume of the aqueous solution of 0.124 M silver fluoride to obtain 17.8 grams of the salt.
What is volume?
To solve this problem, we can use the formula:
mass = molarity x volume x molar mass
where mass is the mass of the solute (in grams), molarity is the concentration of the solution (in moles per liter), volume is the volume of the solution (in liters), and molar mass is the mass of one mole of the solute (in grams per mole).
First, we need to calculate the molar mass of silver fluoride (AgF):
AgF = 1 x 107.87 + 1 x 18.998 = 126.868 g/mol
Next, we can rearrange the formula above to solve for volume:
volume = mass / (molarity x molar mass)
Substituting the given values:
volume = 17.8 g / (0.124 mol/L x 126.868 g/mol)
volume = 117.76 mL
Therefore, we need 117.76 mL of the aqueous solution of 0.124 M silver fluoride to obtain 17.8 grams of the salt.
To know more about molar mass, visit:
https://brainly.com/question/22997914
#SPJ1
Complete question is: 117.76 milliliters of an aqueous solution of 0.124 M silver fluoride is needed to obtain 17.8 grams of the salt.
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
Hydrogen gas + chlorine gas yields hydrogen chloride
Answers
Answer:
\(H_{2} + Cl_{2} --> 2HCl\)
Explanation:
Hydrogen and chlorine are both diatomic, which means they occur in molecular pairs as gases. This means you will have two atoms of each on the left in the reactants, so you will need to add a coefficient of 2 to have 2 atoms of each on the right in the products.
How many moles in 5 grams?
Answers
Answer:
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant
Explanation:
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
There are four molecules of nitrogen and nine molecules of hydrogen present in the diagram.
When the reaction is complete, how many molecules of NH3 are produced?
What is the limiting reactant?
How many molecules of each reactant are remain after the reaction is complete?
Answers
After the reaction is complete, no nitrogen and no hydrogen molecules remain, and 8.00 x 1014 molecules of NH3 are produced.
In the equation, nitrogen and hydrogen react at a high temperature, in the presence of a catalyst, to produce ammonia, according to the balanced chemical equation:N2(g)+3H2(g)⟶2NH3(g)The coefficients of each molecule suggest that one molecule of nitrogen reacts with three molecules of hydrogen to create two molecules of ammonia.
So, to determine how many molecules of ammonia are produced when four nitrogen and nine hydrogen molecules are present, we must first determine which of the two reactants is the limiting reactant.
To find the limiting reactant, the number of moles of each reactant present in the equation must be determined.
Calculations:
Nitrogen (N2) molecules = 4Hence, the number of moles of N2 = 4/6.02 x 1023 mol-1 = 6.64 x 10-24 mol
Hydrogen (H2) molecules = 9Hence, the number of moles of H2 = 9/6.02 x 1023 mol-1 = 1.50 x 10-23 mol
Now we have to calculate the number of moles of NH3 produced when the number of moles of nitrogen and hydrogen are known, i.e., mole ratio of N2 and H2 is 1:3.
The mole ratio of N2 to NH3 is 1:2; thus, for every 1 mole of N2 consumed, 2 moles of NH3 are produced.
The mole ratio of H2 to NH3 is 3:2; thus, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
From these mole ratios, it can be observed that the limiting reactant is nitrogen.
Calculation for NH3 production:
Nitrogen (N2) moles = 6.64 x 10-24 moles
The mole ratio of N2 to NH3 is 1:2; therefore, moles of NH3 produced is 2 × 6.64 × 10−24 = 1.33 × 10−23 moles.
Now, to determine how many molecules of NH3 are produced, we need to convert moles to molecules.
1 mole = 6.02 x 1023 molecules
Thus, 1.33 x 10-23 moles of NH3 = 8.00 x 1014 molecules of NH3 produced.
To find the amount of each reactant remaining after the reaction is complete, we must first determine how many moles of nitrogen are consumed, then how many moles of hydrogen are consumed, and then subtract these from the initial number of moles of each reactant.
The moles of nitrogen consumed = 4 moles × 1 mole/1 mole N2 × 2 mole NH3/1 mole N2 = 8 moles NH3
The moles of hydrogen consumed = 9 moles × 2 mole NH3/3 mole H2 × 2 mole NH3/1 mole N2 = 4 moles NH3
Thus, the moles of nitrogen remaining = 6.64 × 10−24 mol – 8 × 2/3 × 6.02 × 10^23 mol-1 = 5.06 × 10−24 mol
The moles of hydrogen remaining = 1.50 × 10−23 mol – 4 × 2/3 × 6.02 × 10^23 mol-1 = 8.77 × 10−24 mol
Finally, the number of molecules of each reactant remaining can be calculated as follows:
Number of N2 molecules remaining = 5.06 × 10−24 mol × 6.02 × 10^23 molecules/mol = 3.05 × 10−1 molecules ≈ 0 molecules
Number of H2 molecules remaining = 8.77 × 10−24 mol × 6.02 × 10^23 molecules/mol = 5.28 × 10−1 molecules ≈ 0 molecules.
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
did radicals take control in russian revolution
Answers
One of the main political groups at the Petrograd soviet was the Bolsheviks. They were led by Vladimir Lenin, and they believed that the future Russian government should be a Socialist (communist) one.
What do you mean by radical?In chemistry, a molecule with at least on electron is referred to as a radical, sometimes known as a free radical. The majority of molecules have an even variety of electrons, and indeed the c - c single bonds that hold the atoms in a molecule together often comprise of a pair of electrons that the atoms in the bond share.
An extremist is where?A radical is an arithmetic expression that is indicated by the major sign, such as an original number, as you may remember. A radical function is an equation with the independent variable (typically x) acting as the radicand. Radical calculations with either a square root as both the radical are frequently described using square root functions.
To know more about Radicals visit:
https://brainly.com/question/17192138
#SPJ1
can I get some urgent help please?

Answers
Answer:
hi here goes your answer
Explanation:
iv. The lower the PH, the weaker the base
You stay out in the sun too long and get sunburned. What type of energy transfer causes your sunburn?
Group of answer choices
Convection
Conduction
Radiation
Answers
Answer:
Radiation
Explanation:
Calculate the root-mean-square velocity, in m/s, for an oxygen
molecule at 55.2 °C. The universal gas constant, R=8.314 J/mol.K.
Report to three significant figures.
Answers
The root-mean-square velocity of an oxygen molecule at 55.2 °C is approximately 481.1 m/s.
What is root-mean-square velocity?Root-mean-square velocity is the measure of the speed of gas molecules in a gas sample. It is the square root of the average of the squared velocities of the gas particles in a gas sample.
Equation:The root-mean-square velocity of an oxygen molecule can be calculated using the following formula:
v(rms) = √[(3RT)/M]
T = 55.2 °C + 273.15 = 328.35 K
Converting M to kg/mol:
M = 32 g/mol = 0.032 kg/mol
Plugging in the values:
v(rms) = √[(3 x 8.314 J/mol.K x 328.35 K) / 0.032 kg/mol]
v(rms) = 481.1 m/s
To know more about root-mean-square velocity, click here
https://brainly.com/question/31115639
#SPJ9
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

What the mechanisms of action of acidic acid with asprin
Answers
Synthesis. The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group (R-OH → R-OCOCH3).
hope it's help
#carryONlearning