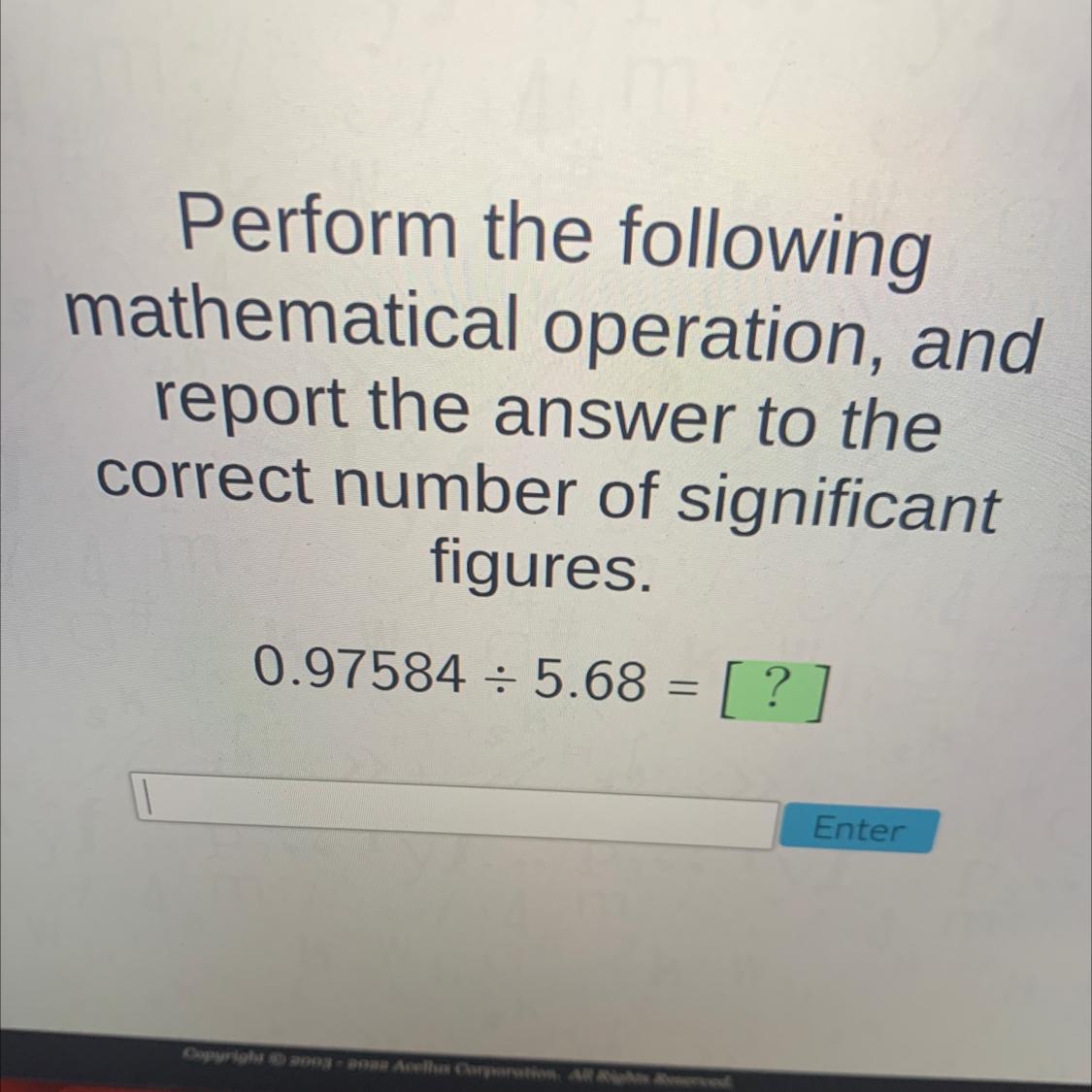
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Related Questions
How do you account for the changes in Co2 without modern man and the burning of fossil fuels?
Answers
To account for changes in CO2 without modern man and the burning of fossil fuels, we need to consider natural processes that affect CO2 levels. Changes in CO2 levels can occur without modern man and the burning of fossil fuels. Natural factors such as volcanic activity, oceanic processes, and the Earth's carbon cycle all play a significant role.
1. Volcanic activity: Volcanoes release CO2 when they erupt. Over millions of years, volcanic activity has significantly contributed to CO2 levels in the atmosphere.
2. Oceanic processes: The oceans act as a carbon sink, absorbing and releasing CO2. Changes in ocean circulation, temperature, and biological activity can affect CO2 levels. For example, during warm periods, the oceans release CO2, while during cold periods, they absorb CO2.
3. Earth's natural carbon cycle: CO2 is naturally exchanged between the atmosphere, land, and oceans through processes like photosynthesis, respiration, and decay. Changes in these processes can influence CO2 levels.
In conclusion, changes in CO2 levels can occur without modern man and the burning of fossil fuels. Natural factors such as volcanic activity, oceanic processes, and the Earth's carbon cycle all play a significant role. It's important to understand these natural processes when studying CO2 variations throughout history.
To know more about fossil fuels, visit:
https://brainly.com/question/2582135
#SPJ11
A tank contains a mixture of 3.00 mol N₂, 2.00 mol O₂, and 1.00 mol CO₂ at 25 °C and a total pressure
of 10.0 atm. Calculate the partial pressure of each gas in the mixture.
Answers
The partial pressure of N₂ is 3.75 atm, the partial pressure of O₂ is 2.50 atm, and the partial pressure of CO₂ is 1.25 atm in the given mixture at 25 °C and a total pressure of 10.0 atm.
To calculate the partial pressure of each gas in the mixture, we can use the concept of Dalton's law of partial pressures. According to this law, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas.
First, we need to find the mole fraction of each gas in the mixture. The mole fraction of a gas is the ratio of the number of moles of that gas to the total number of moles in the mixture. We can calculate the mole fraction using the following formula:
Mole fraction (X) = Moles of gas / Total moles of all gases
For N₂:
Mole fraction of N₂ (X_N₂) = 3.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.375
For O₂:
Mole fraction of O₂ (X_O₂) = 2.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.250
For CO₂:
Mole fraction of CO₂ (X_CO₂) = 1.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.125
Next, we can use the mole fractions to calculate the partial pressures of each gas. The partial pressure of a gas is equal to the mole fraction of that gas multiplied by the total pressure of the mixture.
Partial pressure of N₂ (P_N₂) = X_N₂ * Total pressure = 0.375 * 10.0 atm = 3.75 atm
Partial pressure of O₂ (P_O₂) = X_O₂ * Total pressure = 0.250 * 10.0 atm = 2.50 atm
Partial pressure of CO₂ (P_CO₂) = X_CO₂ * Total pressure = 0.125 * 10.0 atm = 1.25 atm
For more such questions on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
heating of glass until it melts what change is it
Answers
Answer:
physcial change
Explanation:
As it has visible changes on shape and size
The table above shows the structural formulas and molar masses for three different compounds. Which of the following is a list of the compounds in order of increasing boiling points?
A. Butane < 1-propanol < acetone
B. Butane < acetone < 1-propanol
C. 1-propanol < acetone < butane
D. Acetone = butane < 1-propanol

Answers
Butane < acetone < 1-propanol is a list of the compounds in order of increasing boiling points.
Which substance has a greater boiling point?The intermolecular interactions between molecules in a compound play a major role in boiling point. Higher boiling temperatures are a result of greater intermolecular interactions, bigger masses, and less branching.
The highest boiling point is for HF. As we go from HF to HI, the van der Waals forces of attraction between all hydrogen halides get stronger.
Boiling point rises as the difference in electronegativity grows. Additionally, the bp grows as the molecule's size does. So the sequence is CO2, CS2, CCl4, and then H2O.
Van der Waals attraction and dipole-dipole attraction draw acetone molecules together.
To learn more about compounds refer to:
https://brainly.com/question/26487468
#SPJ1
3. The chemical formula of a mineral can be considered a statement about the chemical components and their proportions in a mineral's structure. One of the basic tenets is that the mineral must be electrically neutral. For each of the minerals listed below, write down the mineral formulae and list the valence (oxidation) state of cations and anions that make up that mineral.
2 | Page
EASC 219: Mineralogy Fall 2022
a. uvarovite
b. azurite
c. cuprite
d. gypsum
e. galena
Answers
The valence states provided are general representations and may vary depending on specific conditions and coordination environments.
a. Uvarovite: The mineral formula for uvarovite is Ca3Cr2(SiO4)3. In this formula, the valence state of calcium (Ca) is +2, the valence state of chromium (Cr) is +3, and the valence state of silicon (Si) is +4. Oxygen (O) is usually assigned a valence state of -2.
b. Azurite: The mineral formula for azurite is Cu3(CO3)2(OH)2. In this formula, the valence state of copper (Cu) is +2, carbonate (CO3) has a valence state of -2, and hydroxide (OH) has a valence state of -1.
c. Cuprite: The mineral formula for cuprite is Cu2O. In this formula, the valence state of copper (Cu) is +1, and oxygen (O) is usually assigned a valence state of -2.
d. Gypsum: The mineral formula for gypsum is CaSO4·2H2O. In this formula, the valence state of calcium (Ca) is +2, sulfur (S) has a valence state of +6, and oxygen (O) is usually assigned a valence state of -2. The water molecules (H2O) do not have a net charge.
e. Galena: The mineral formula for galena is PbS. In this formula, the valence state of lead (Pb) is +2, and sulfur (S) has a valence state of -2.
It's important to note that the valence states provided are general representations and may vary depending on specific conditions and coordination environments.
Learn more about valence from below link
https://brainly.com/question/371590
#SPJ11
which are the most critical parameters to control the oxide growth. why?
Answers
The following are the most critical parameters to control oxide growth: Temperature: The temperature at which oxide growth occurs is critical to the process.
The rate of oxide growth varies exponentially with temperature. For most materials, the oxidation rate doubles every 10°C increase in temperature. Ambient gas: The composition of the ambient gas and its partial pressure are critical to oxide growth. The oxygen concentration in the atmosphere affects oxide growth. The oxidation rate can be significantly reduced if the oxygen concentration is reduced or removed from the atmosphere. Metal composition: The material composition of the substrate to be oxidized has an impact on the oxidation rate.
Aluminum oxide, for example, grows more rapidly on pure aluminum substrates than on aluminum alloys. Oxidation can be significantly impacted by the presence of impurities or alloying elements.
Therefore, temperature at which oxide growth occurs is critical to the process.
To learn more about substrate check the link below-
https://brainly.com/question/4047091
#SPJ11
Which of the following is true about electrostatic forces? Check all that are true:
Like Gravity, electrostatic forces always attract
An atom with an equal number of protons and electrons can pull with an electrostatic force
An ion with a negative charge will repel another ion with a negative charge
Electrostatic forces are a fundamental force in the universe
Answers
Answer:
Option 3 or C An ion is all in Electrostatic forces so it's c if its not I'll work it out for you
An ion with a negative charge will repel another ion with a negative charge is the correct statement.
What is electrostatic forces?The electrostatic force is the force that exists between electrically charged particles or objects at rest means that objects having similar charge repel each other whereas objects having different charges attract each other.
An ion with a negative charge will repel another ion with a negative charge is the statement true about electrostatic forces because electrostatic force always occur between static object having charges on it.
Learn more about force here: https://brainly.com/question/12970081
identify each of the following as alpha decay, beta decay, positron emission, or gamma emission: (5.1, 5.2) c55127s→ x54127e e 10 s3890r→ y3990 −10e a85218t→ b83214i h24e
Answers
There is insufficient data from the first process (c55127s x54127e) to identify the kind of decay.
Positron emission is demonstrated in the second procedure (e10s3890r y399010e).
There is insufficient data from the third process (a85218t b83214i) to identify the kind of decay.
Gamma emission is demonstrated by the fourth process (h24e).
Let's examine each of the steps mentioned:
(5.1, 5.2) c55127s → x54127e
In this procedure, an atomic nucleus with the sign Cs-127 is changed into an alternative nucleus with the symbol Xe-127. However, the information offered does not specify the precise type of decay. Without more information, it is impossible to say whether it involves positron emission, gamma emission, beta decay, or alpha decay.
e10s3890r → y3990−10e
This reaction releases a positron while changing an atomic nucleus with the sign Sr-90 into another nucleus with the symbol Y-90. Positron emission can be seen in this instance.
a85218t → b83214i
An atomic nucleus with the sign At-218 is changed into another nucleus with the symbol Bi-214 throughout this procedure. However, the information offered does not specify the precise type of decay. Without more information, it is impossible to say whether it involves positron emission, gamma emission, beta decay, or alpha decay.
h24e
A high-energy photon (gamma ray) is released from an atomic nucleus during this process. Gamma emission can be seen in this instance.
To sum up:
There is insufficient data from the first process (c55127s x54127e) to identify the kind of decay.
Positron emission is demonstrated in the second procedure (e10s3890r y399010e).
There is insufficient data from the third process (a85218t b83214i) to identify the kind of decay.
Gamma emission is demonstrated by the fourth process (h24e).
To know more about alpha decay:
https://brainly.com/question/33462714
#SPJ4
A student describes the motion of particles in each of the four different states of matter. State 1: Particles that are charged move freely State 2: Particles move freely at high speed State 3: Particles are locked in place State 4: Particles slide past one another Which state describes plasma? Group of answer choices State 1 State 2 State 3 State 4
Answers
Answer:
state 1
Explanation:
Particles that are charged move freely State describes the state describing plasma. Stage 1 is correct.
What is plasma?Plasma is an ionized gas in which the ions that are positively charged can move freely and electrons can also move freely with low pressure and high temperature in the gaseous state of the matter.
In plasma, there are only charged particles present that can only move freely with no pressure it is an ionized gas because the formation of gas is done after only the ionization process and these ionized particles can only move.
Therefore, Stage 1 is correct. Particles that are charged move freely State describes the state describing plasma.
Learn more about plasma , here:
https://brainly.com/question/18207038
#SPJ2
can anyone tell me "what are the exceptions of law of reciprocal proportions??"
Answers
the vapor pressure of pure water at 308 k is 5571 pa. the vapor forms an ideal gas. 1) in some oil, the equilibrium concentration of water molecules is only 1% as large as in pure water. suppose that the pure water is covered with a layer of that oil. what's the equilibrium vapor pressure of water above the oil layer?
Answers
In this particular question, the equilibrium vapor pressure of water above the oil layer is 55.71 Pa.
Give a brief detail about vapor pressure.
The pressure exerted by a vapour in thermodynamic equilibrium with its condensed phases (solid or liquid) at a specific temperature in a closed system is referred to as vapour pressure (or vapour pressure in English-speaking nations other than the US; see spelling variants). A liquid's evaporation rate can be determined by looking at the equilibrium vapour pressure. It has to do with how often particles tend to float away from liquids (or a solid). Volatile is a term used to describe a chemical that has a high vapour pressure at room temperature. Vapor pressure is the force that vapour exerts when it is present above a liquid surface.
To learn more about vapour pressure
https://brainly.com/question/2693029
#SP J4
gene vincent and eddie cochran were particularly popular with:
Answers
Gene Vincent and Eddie Cochran were particularly popular with teenagers and young adults during the 1950s.
Gene Vincent and Eddie Cochran were American rock and roll musicians who gained popularity in the 1950s. They were part of the rockabilly movement, which combined elements of country music with rhythm and blues.
Gene Vincent was known for his hit song 'Be-Bop-A-Lula,' which became a rock and roll classic. Eddie Cochran was known for his energetic performances and songs like 'Summertime Blues' and 'C'mon Everybody.'
Both artists had a significant impact on the development of rock and roll music and were particularly popular with teenagers and young adults during their time.
Learn more:About Gene Vincent here:
https://brainly.com/question/30270663
#SPJ11
Gene Vincent and Eddie Cochran were particularly popular with the youth and rock and roll enthusiasts of the late 1950s and early 1960s.
Gene Vincent and Eddie Cochran were particularly popular with the youth and rock and roll music enthusiasts of the late 1950s and early 1960s. Their energetic performances and rebellious image resonated with the emerging teenage audience at the time.
They were influential figures in the rockabilly and rock and roll genres, known for their hits such as "Be-Bop-A-Lula" by Gene Vincent and "Summertime Blues" by Eddie Cochran. Their music and style captured the spirit of youthful rebellion and played a significant role in shaping the early rock and roll era.
Gene Vincent and Eddie Cochran were influential figures in the rock and roll music scene of the late 1950s and early 1960s. They were particularly popular with the teenage audience of the time, as their music and persona embodied the rebellious and energetic spirit of the youth culture.
Gene Vincent, born Vincent Eugene Craddock, rose to fame with his hit song "Be-Bop-A-Lula" in 1956. Known for his distinctive vocal style and wild stage presence, Vincent became a rockabilly icon. His music blended elements of rock and roll, rhythm and blues, and country, creating a unique sound that resonated with young listeners. Songs like "Bluejean Bop" and "Race with the Devil" further solidified his popularity.
Eddie Cochran, on the other hand, was a multi-talented musician, singer, and songwriter. He gained fame with his upbeat and catchy songs, such as "Summertime Blues" and "C'mon Everybody." Cochran's music was characterized by his skillful guitar playing, heartfelt lyrics, and a distinctive rock and roll sound. His contributions to the genre and his early death at the age of 21 in a tragic car accident solidified his status as a rock and roll legend.
Both Gene Vincent and Eddie Cochran were known for their electrifying live performances and their impact on the rock and roll genre. Their music resonated with young audiences who were seeking an outlet for their rebellious spirit and love for energetic, guitar-driven music. Their influence can still be felt in the development of rock music and the inspiration they provided to subsequent generations of musicians.
To know more about enthusiasts refer here
https://brainly.com/question/37825138#
#SPJ11
Sciences Grad Bosch Law of Conservation of Mass - Assess it
contents
Target: 10/2001
Science and Enging
Achemical reaction takes place in a closed system. The mass of the reactants before the reaction was
55 watte mass of the products of the reaction be according to the law of conservation
Properties of Matter
Answers
Answer:
55
Explanation:
According to the law of conservation properties of Matter in a closed system the mass of reactants is equal to the mass of products. Because there is no lose of matter or energy in closed system
Compounds are held together by chemical bonds, can be represented by a chemical
formula,
__(can or cannot) be broken down by a physical process, and are made
up of ___________ or more different elements.
cannot, two
can, two
cannot, one
can, one
Answers
Answer:
Cannot, two
Explanation:
The component elements of a compound can only be separated via a chemical reaction that breaks the atomic bonds that bind its molecules. Also, compounds are formed by 2 or more different elements.
Malachite is a green colored mineral that is 57.5% copper. What mass of copper is present in a 250.0 g sample of malachite
Answers
57.5% Cu2(CO3)(OH) (OH) Other elements are also present in addition to copper.
To get the mass percent of copper in malachite (Cu2(OH)2CO3), apply the following formula:
Malachite (Cu2(OH)2CO3) has a molar mass of 2x63.5% + 2(16+1) + 12 + (16x3) = 127 + 2(17) + 12 + 48 = 127 + 34 + 12 + 48 = 221g/mol.
Cu2(OH)2CO3 has a Cu mass of 2 x 63.5g, or 127g.
The mass-based fraction of Cu in Cu2(OH)2CO3 is calculated as Cu2(OH)2CO3 mass per mol mass multiplied by 100 = 127/221 x 100 = 57.5%.
Malachite Cu2(OH)2CO3 has 57.5% of its mass in copper.
To learn more about Malachite's please click on below link
https://brainly.com/question/15461613
#SPJ4
Coca - Cola and Pepsi suggest that their products taste best when chilled to around 40° F . Why might this be ?
Answers
The reason Coca-Cola and Pepsi suggest that their products taste best when chilled to around 40°F is due to a combination of factors related to human taste preferences and the chemical properties of the drinks themselves.
Firstly, colder temperature reduce our perception of sweetness, which can make beverages taste less cloying and more refreshing. Additionally, carbonated drinks like Coke and Pepsi release more carbon dioxide gas when they are chilled, which creates a tingly sensation on the tongue that many people find pleasurable. Finally, colder temperatures can help mask any off-flavors or bitter notes in the drinks, which can improve overall enjoyment.
From a chemical perspective, the cold temperature can also help to preserve the carbonation and prevent the drink from going flat too quickly. So, while Coca-Cola and Pepsi may taste perfectly fine at room temperature, they are designed to be enjoyed at a cooler temperature to provide the best sensory experience. Ultimately, taste is a subjective experience, and some people may prefer their soda at different temperatures, but the suggested serving temperature is based on science and research to provide the optimal taste experience for the majority of consumers.
learn more about temperature Refer: https://brainly.com/question/31792425
#SPJ11
Identify the number of significant figures in each of these measurements of an object length: a. 76.48 cm b. 76.47 cm c. 76.59 cm
Answers
All of the measurements have 4
The number of significant figures in each of these measurements of an object's length: a. 4 b. 4 c. 4.
What are significant figures?The significant figures are the digits or numbers from zero to nine which are used to signify and specify the reporting of the measurement having uncertain digits.
Starting zero is not significant, decimal is not significant, ending zero after the decimal is significant and ending zero before and without decimal is not significant.
Therefore, a. 76.48 cm = 4 b. 76.47 cm c. = 4 76.59 cm = 4 are the number of significant figures in each of these measurements of an object's length.
Learn more about significant figures, here:
https://brainly.com/question/14359464
#SPJ2
be able to explain the chemistry behind the edta titrations. why do we need the buffer? why do we spike the samples with mgedta? write the reactions to help explain. o
Answers
A buffer is used to maintain a constant pH during the titration process for accurate results. Spiking the samples with MgEDTA helps to control the pH and provides a known concentration of EDTA for the titration.
EDTA titrations are commonly used in analytical chemistry to determine the concentration of metal ions in a solution. The principle behind this technique lies in the ability of EDTA to form stable complexes with metal ions. EDTA is a hexadentate ligand, meaning it can coordinate with a metal ion using six of its electron-pair-donating sites.
During the titration, a buffer solution is essential to maintain a constant pH. This is crucial because the formation of metal-EDTA complexes is pH-dependent. A slight deviation in pH can affect the stability of the complex and lead to inaccurate results. The buffer resists changes in pH by neutralizing any added acids or bases, providing a stable environment for the titration.
To ensure accurate measurements, the samples are spiked with MgEDTA. Spiking involves adding a known concentration of a standard compound to the sample. In this case, MgEDTA is added, which releases free EDTA in the solution. The purpose of spiking is two-fold: first, it helps control the pH by providing a known concentration of EDTA, and second, it allows for calibration and standardization of the titration method.
The reaction between EDTA and metal ions can be represented by the following general equation:
\(Mn^+ + EDTA = M(EDTA)^-\)
Where \(Mn^+\) represents the metal ion and\(M(EDTA)^-\) is the resulting metal-EDTA complex. The stability constant of the complex determines the equilibrium position, which is affected by pH.
Overall, understanding the chemistry behind EDTA titrations, the role of buffers, and the purpose of spiking samples with MgEDTA helps ensure accurate and reliable results in metal ion analysis.
Learn more about EDTA titrations here:
https://brainly.com/question/30667405
#SPJ11
Plan an investigation to explore the relationship between properties of substances and the electrical forces within those substances.
What can properties of substances tell us about the electrical forces within those substances?
In this activity, you will plan and conduct an investigation to compare a single property across several substances. You must select a measurable property, such as boiling point or surface tension. After your investigation, you will compare the results and use your data to make inferences about the strength of the electrical forces in each substance you tested
Answers
The first step in this investigation will be to select several substances to test. It is important to choose substances that have similar chemical composition but differ in physical properties.
Once the substances have been selected, the next step is to measure the single property across each of the substances. This can be done through a variety of methods, such as using a thermometer to measure boiling points or a microscope to measure surface tension.
After the data has been collected, it should be compared and analyzed to determine how the property is related to the strength of the electrical forces in the substance. By comparing the data and making inferences, it is possible to determine how properties of substances can indicate the strength of the electrical forces within those substances.
Know more about Thermometer here
https://brainly.com/question/24189042#
#SPJ11
9) Is there any other ratio of aluminum and oxygen ions that could exist?
For instance, could you have Alz0 or AlO2? Explain your answer.
Answers
Answer:
The ratio of aluminium and oxygen ions that only exists is 2:3
Since Aluminium has 3 valence electrons and oxygen has 2 vacant orbitals
Aluminium holds a valence of 3 and oxygen 2, when they react a compound of formula
\(Al _{2} O _{3}\)
is formed
According to the electronic configuration and valency, ratio that could exist between aluminium and oxygen is 2:3.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ2
If 130.0 g of steam at 200.0 °C absorbs 900.0 kJ of heat energy, what will be its increase in temperature?
Help pleaseeeeee

Answers
Answer:
t=1855.45 C
Explanation:
m=0.13kg t1=200C H=900000J Cp=4182J/kgC
h=m*cp*change in temperature
900*10^3=0.13*4182*(T-200)
1655.45=t-200
t=1855.45 C
Atomic Radius: Complete the following:
#of Atomic A) Which element has the smaller atomic radius?
Element
Protons Radius
B) Why do you think this element has a smaller radius? (how
Be
4
153
does the number of protons affect atomic radius?)
F
147
lonization Energy: Complete the following:
Atomic
Element
1st lonization A) Which element has the smaller 1st ionization energy?
Radius Energy B) Why do you think this element has a smaller 1st ionization
He
energy? (how does atomic radius affect 1st ionization energy?)
Kr
Electronegativity: Complete the following:
# of
Element
Electroneg- A) Which element has the larger electronegativity?
Protons ativity
B) Why do you think this element has a larger electronegativity?
(how does # of protons affect electronegativity?)
Ga

Answers
Answer:
2 A): Fluorine
B): more protons, more force attraction less atomic radius
3A): Helium
B):it has small atomic radius
4 th question is not visible.....
A radioactive substance decreases by 65% each hour. Find the hourly decay factor. The hourly decay factor is__
Answers
A radioactive substance decreases by 65% each hour. Find the hourly decay factor. The hourly decay factor is 0.35.
Chemicals in the class of radionuclides (also known as radioactive materials) have unstable atomic nuclei. They become stable by undergoing modifications in the nucleus (spontaneous fission, alpha particle emission, neutron conversion to protons, or the opposite).
A radioactive atom will naturally emit radiation in the form of energy or particles in order to transition into a more stable state. The difference between radioactive material and the radiation it emits must be made.
Learn more about radioactive, here:
https://brainly.com/question/18917411
#SPJ1
PLEASE! I HAVE 20 MINS LEFT :( Two aqueous solutions of AgNO3 and NaCl are mixed. Which of the following diagrams best represents the mixture? For simplicity, water molecules are not shown (Ag + = gray, Cl- = orange, Na + = green, NO ^ - 3 = blue) PLEASE I NEED HELP I ONLY HAVE 15 MINS PLS :'((

Answers
Answer:
diagram C best represents the mixture
How does the Pauli exclusion principle explain the periodic
table. Please explain in detail.
Answers
The Pauli exclusion principle explains the periodic table by stating that no two electrons in an atom can have the same set of quantum numbers.
In more detail, the periodic table organizes elements based on their atomic number, which represents the number of protons in an atom's nucleus. Each element consists of a unique arrangement of electrons around the nucleus. The Pauli exclusion principle, formulated by Wolfgang Pauli, states that within an atom, no two electrons can have the same set of quantum numbers.
Quantum numbers describe various properties of electrons, such as their energy, orbital shape, and orientation. According to the principle, each electron must have a distinct combination of quantum numbers, including the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). This means that in a given atom, electrons occupy different energy levels and subshells, contributing to the observed patterns in the periodic table. The principle helps explain the filling order of atomic orbitals and the organization of elements into periods and groups based on their electronic configurations. It also plays a crucial role in understanding chemical bonding and the properties of elements.
Learn more about periodic table here: brainly.com/question/28747247
#SPJ11
All stars are composed of a mixture of elements. When these elements are heated they emit specific amounts of electromagnetic radiation, known as an emission spectrum. Each element emits a unique, identifiable, spectrum.
Using the Bright-line emission spectrum chart below, identify the elements present in this modeled star (found in the line labeled "mixture").
Lithium and cadmium are in the mixture. Strontium is not in the mixture.
Lithium and strontium are in the mixture. Cadmium is not in the mixture
Cadmium and strontium are in the mixture. Lithium is not in the mixture
All of the shown elements are present in the mixture.
Answers
Lithium and cadmium are in the mixture. Strontium is not in the mixture. Cadmium and strontium are in the mixture. Lithium is not in the mixture
When a star's core runs out of hydrogen, it starts to fuse helium to create increasingly heavier elements, like carbon and iron. The star either erupts into a supernova as its fuel runs out, releasing those elements into space, or it violently collapses, forming neutron stars as well as black holes.
For the first time, researchers have demonstrated that certain of the heavier elements of the periodic table are produced when combinations of neutron stars collide violently and erupt. Light elements like helium and hydrogen were created during in the big bang, and stars' cores use fusion to create elements up to iron.
To know more about mixture of element from the given link
https://brainly.com/question/29588376
#SPJ4
When temperature increases, the rate at which glucose is used by cells is expected to... A) decrease B) Increase C) Stay the Same
Answers
B) Increase.
When the temperature increases, the rate at which glucose is used by cells is expected to increase.
What is glucose?
Glucose is a monosaccharide that is essential for the metabolic processes of most living organisms.
It's an important energy source for all cells in all organisms.
When glucose is consumed by cells, it goes through a series of chemical reactions, including glycolysis and the citric acid cycle, that produce ATP, which the cells use for energy.
What effect does temperature have on the rate of glucose utilization by cells?
Temperature has a significant impact on the rate of glucose utilization by cells.
This is due to the fact that most biological processes are enzyme-mediated and enzymes are highly temperature sensitive.
The rate of most enzymatic reactions increases with temperature, which means that higher temperatures will result in more glucose being used by cells.
As a result, if the temperature increases, the rate at which glucose is used by cells is expected to increase.
Therefore, the correct answer is option B) Increase.
Learn more about glucose from this link:
https://brainly.com/question/397060
#SPJ11
Assume that a proton is scalar coupled (J-coupled) to proton(s) with different chemical environments. If this proton shows a triplet signal, how many proton(s) is it scalar coupled to
Answers
If a proton shows a triplet signal, it is scalar coupled to two protons with different chemical environments.
If a proton shows a triplet signal, it means that it is coupled to two protons with different chemical environments. The triplet signal arises from the splitting of the central proton's signal into three peaks of equal intensity by the J-coupling interaction with the adjacent protons.
The two adjacent protons must be in different chemical environments for the central proton to show a triplet signal. This is because the J-coupling constant (J) is dependent on the distance between the coupled protons and the nature of the chemical bond that connects them. If the adjacent protons were in the same chemical environment, they would experience the same J-coupling constant, and the central proton would show a doublet signal.
Therefore, if a proton shows a triplet signal, it is scalar coupled to two protons with different chemical environments.
Learn more about proton Visit: brainly.com/question/12748782
#SPJ4
Which of the following instrument would you use to see a plant cell?
a. Kaleidoscope
C. Periscope
b. Microscope
D. Telescope

Answers
this is the answer .
Which statements are true about the rainforest climate? (Select all that apply.)
They have low temperatures.
They receive a lot of precipitation.
They receive little precipitation.
They have high temperatures.
Answers
Answer:Answer:They have high temperatures and They receive a lot of precipitation.
Explanation:
Answer:
They receive a lot of precipitation. They have high temperatures.
Explanation: