Please help ASAP
Which of the following describes a solute?
A.
a substance suspended in a liquid
B.
a substance dissolved in a solution
C.
a substance that dissolves another substance in a solution
D.
a substance that forms a colloid in a liquid
Answers
Answer:
B
Explanation:
Answer:
B thats kinda funny that i just had that question
Explanation:
Related Questions
A bond between two iodine atoms would have what as the percent ionic character
A. 53%
B. 83%
C. 0%
D. impossible to calculate
Answers
Answer:
Your answer should be 53%
I'm very sorry if I'm wrong I have not done this in forever
Explanation:
No explanation
URGENT PLEASE HELPIf 25 grams of liquid water releases 75J of heat when water cools from 88 degrees Celsius what is the final temperature?
Answers
Answer:
HEY RUBY!! WE ARE BOTH FAILING!!!!!!!
Explanation:
#&-78$#'☠️
explain why the troposphere has a larger total mass in the stratosphere even though the stratosphere so much bigger
Answers
Answer:
The troposphere is the lowest layer of Earth's atmosphere, and is also where nearly all weather conditions take place. It contains 75% of the atmosphere's mass and 99% of the total mass of water.
Explanation:
The process of ice making usually occurs in which temperature range?
A. -10*F to 0*F
B. 0*F to 5*F
C. 5*F to 20*F
D. 20*F to 30*F
Answers
The range of -10°F to 0°F is the ideal temperature range for ice-making because it promotes faster freezing time and produces high-quality ice.
The process of ice-making usually occurs in the temperature range of -10°F to 0°F. Ice making is the process of making ice from water. The process is usually used in refrigerators, air conditioning units, and in manufacturing industries. During this process, water is changed from its liquid state to a solid state through a cooling process. The ice-making temperature range is important because it determines how long it will take for water to freeze into ice at a given temperature range. The colder the temperature, the quicker the ice-making process. Therefore, the range of -10°F to 0°F is the ideal temperature range for ice-making because it promotes faster freezing time and produces high-quality ice.
To know more about refrigerators visit :
brainly.com/question/13002119
#SPJ11
what is CLF full form
Answers
Answer:
compact fluorescent light
A ____________ is a property, the expansion, redevelopment, or
reuse of which may be complicated by the presence or potential
presence of a hazardous substance, pollutant, or contaminant.
Answers
A brownfield is a property, the expansion, redevelopment, or reuse of which may be complicated by the presence or potential presence of a hazardous substance, pollutant, or contaminant.
A “brownfield” generally refers to a parcel of land that was previously used for industrial purposes and which is contaminated by low concentrations of hazardous chemicals.
A brownfield development requires more work and investment upfront: existing structures may have to be demolished, materials must be removed, and developers may have to engage in extensive environmental cleanup to remove pollutants.
Learn more about Brownfield land, here:
https://brainly.com/question/3762221
#SPJ4
______elements that have a longer half-life are more _______ than those that have a shorter half-life.
Answers
Answer:
Radioactive elements
Stable
Explanation:
Radioactive elements that have a longer half-life are more stable than those that a shorter half-life.
Half-life is the time taken for half of a radioactive element to decay by half of its original composition.
Now, if a substance have a long half-life, it is more stable and takes time to break down. For atoms with a short life, they are highly unstable and breaks down readily and easily.
Why does the carbon anode burn away in the electrolysis of aluminium chloride?
Answers
Germanium has a face-centered cubic unit cell. The density of germanium is 5.32 g/cm3. Calculate a value for the atomic radius of germanium.
Answers
Answer:
1.59x10⁻¹⁰m
Explanation:
To solve this question we must know that the length of the cubic cell, X, is equal to:
X = √8 * R
Where R is the atomic radius of germanium
And that in 1 unit cell there are 4 atoms of germanium.
To solve this question we must find the mass in 1 unit cell, with this mass we can find the volume of the cube and the length. With the length we can know the atomic radius:
Mass in 1 unit cell -Molar mass Ge = 72.64g/mol:
4 atoms Ge * (1mol / 6.022x10²³ atoms) = 6.64x10⁻²⁴ moles Ge
6.64x10⁻²⁴ moles Ge * (72.64g / mol) = 4.825x10⁻²²g Ge
Volume unit cell:
4.825x10⁻²²g Ge * (1cm³ / 5.32g) = 9.07x10⁻²³cm³
Length unit cell:
∛9.07x10⁻²³cm³ = 4.49x10⁻⁸cm * (1m / 100cm) = 4.49x10⁻¹⁰m
Atomic radius Ge:
4.49x10⁻¹⁰m / √8 =
1.59x10⁻¹⁰mPlease answer my questions. I really need them soon. I will post the links for other questions in the comments.

Answers
Explanation:
option option B is the correct answer of given statement helium-4(He)=2
What should a simplified model of a large molecule like glucose show?
Answers
Glucose is the simplest sugar and carbohydrate that provides energy. The simplified model of glucose (C₆H₁₂O₆) shows carbon, hydrogen, and oxygen atoms linked together.
What is glucose?Glucose is an example of a carbohydrate macromolecule that is further classified as a monosaccharide. They are crystalline and fundamental units of carbohydrates.
The molecular formula of glucose is C₆H₁₂O₆ and the mass is 180.156 g/mol. It is an aldohexose that contains an aldehydic functional group. In its structure, there are six oxygen atoms, six carbon atoms, and twelve hydrogen atoms.
Therefore, the glucose molecule is composed of C, H, and O.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
What is one of the most common uses of polyvinyl chloride? select one: a. 2-l soda bottles b. styrofoam cups c. plastic pipes d. plastic food bags e. plastic garbage cans
Answers
The correct answer choice which is the most common use of polyvinyl chloride is: plastic pipes. Option C
Below are some other uses of polyvinyl chloride:
It's pipes is used for fittingsIt's pipes is used for building infrastructure and structural materialIt is used for coatingsWhat is polyvinyl chloride?Polyvinyl Chloride (PVC) is polymer which is used in various applications including widespread use in building, transport, packaging, electrical and healthcare applications.
So therefore, the most common use of polyvinyl chloride is in the making of plastic pipes
Learn more about polyvinyl chloride:
https://brainly.com/question/26356455
#SPJ1
Copper metal (Cu) reacts with silver nitrate (AgNOg) in aqueous solution to form Ag and Cu(NO3)2. The balanced chemical equation is shown below. Cu+2AgNO3 Cu(NO3)2 + 2Ag The molar mass of Cu is 63.5 g/mol. The molar mass of Ag is 107.9 g/mol. What mass, in grams, of Ag is produced from a reaction of 31.75 g of Cu? O 26.95 O107.9 O 215.91 431.82
show your work pls
Answers
Answer:
107.9
Explanation:
Ionic bond is formed between atoms of ____and ______
A. metals, non-metals
B. metals, metals
C. non-metals, non-metals
Answers
Answer:
A. metals and non-metals
Answer:
Metals-Non-Metals
Explanation:
You see, that is the answer because when the asnwer.
\(\geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq \geq\)
In the chemical equation A + B ⇔ C + D, which of the chemicals would be termed the reactant(s)?
A) A only
B) B only
C) A and B
D) C and D
E) C only
Answers
Correct answer is A and B. The reactant(s) in the chemical equation A + B ⇔ C + D would be option C, A and B.
A chemical reaction's reactants are the substances that take part in it. A chemical reaction is the term used to describe how atoms, which are the basic building blocks of matter, rearrange themselves to create new combinations. Reactants are raw materials that react with one another.
In the chemical equation A + B ↔ C + D, the reactants are the chemicals that participate in the reaction to form the products. In this case, the reactants are A and B.
To know more about chemical equation visit:-
https://brainly.com/question/30087623
#SPJ11
Plss answer the question in the picture.

Answers
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.
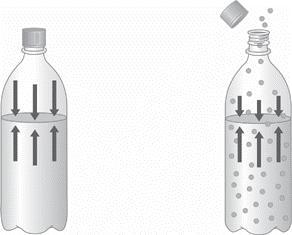

Answers
Answer:
SEE EXPLANATION
Explanation:
If i refer to the bottle at the left hand side as A and the bottle at the right hand side as B. I can say that the bottle A contains solutes which are more soluble than the solutes in bottle B. consequently, A has a lower vapour pressure than B.
We can also see that the pressure in A is much higher than in B(as indicated by the length of the arrows). The higher the pressure, the greater the solubility of the gas. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Again let me call the beaker on the left A and the beaker on the right B. We can see that the beaker on the left contains 10 ml of solution while the beaker on the right contains 50 ml of solution. It follows that beaker A contains a more concentrated solution since that much amount of solute is contained in a smaller volume than in beaker B as shown in the image.
true or false: radiation can be detected because of its green glow, intense heat, crackling sound and ammonia smell.
Answers
False.
Radiation itself does not typically have a green glow, intense heat, crackling sound, or ammonia smell. These descriptions do not accurately represent the properties of radiation.
The emission of energy in the form of particles or electromagnetic waves is referred to as radiation. Our senses cannot immediately notice it. Radiation is measured and detected using specialized apparatus and detectors.
Alpha particles, beta particles, gamma rays, and X-rays are a few examples of different forms of radiation that have unique characteristics and may be identified with the right tools. For instance, ionizing radiation is typically detected using Geiger-Muller counters or scintillation detectors, whereas radiation exposure is measured using dosimeters.
For precise radiation risk identification and protection, it's crucial to rely on the right detection tools and follow safety procedures.
To know more about radiation:
https://brainly.com/question/31106159
#SPJ4
A sample of gas at a fixed pressure has a temperature of 300 K and a volume of 3 L. Calculate the volume if the gas is heated to 700 K.
Answers
Answer:300k=3L
700k=x
cross multiply
300x=2100; x=7L
Explanation:
Answer:
7L
Explanation:
In the sixteenth century, the geocentric theory was a daim with substantial evidence. Which of the following best describes why scientists began to regard the heliocentric theory as more acceptable?
Answers
Answer:
The heliocentric theory was better supported by data explaining the rotation of the planets and other bodies in the solar system.
Explanation:
how many grams are in the 3 moles of HF
Answers
There are 60 grams in 3 moles of HF.
One mole of a substance contains a specific number of particles, called Avogadro's number. This number is approximately 6.022 × 10²³ particles per mole.
Therefore, the mass of one mole of a substance can be determined from its molar mass.
For example, the molar mass of HF (hydrogen fluoride) is approximately 20 g/mol (1.008 g/mol for hydrogen + 18.998 g/mol for fluorine).
Therefore, one mole of HF weighs 20 grams.
To determine the mass of 3 moles of HF, multiply the molar mass by the number of moles:3 mol HF × 20 g/mol HF = 60 g HF
Therefore, there are 60 grams in 3 moles of HF.
For more such questions on moles visit:
https://brainly.com/question/29367909
#SPJ8
Which substance has a standard enthalpy of formation of zero?.
Answers
Answer: In its natural condition, a pure element
.For which of the following reactions is delta S at 25 degrees Celsius closest to zero?
A) C2H4 (g) + Br2 (l) -> C2H4Br2 (l)
B) H2 (g) + I2 (s) -> 2HI (g)
C) N2 (g) + O2 (g) -> 2NO (g)
D) CH3CHO (g) + 5/2O2 (g) -> 2CO2 (g) + 2H2O (g)
E) 2NO (g) + O2 (g) -> 2NO2 (g)
Answers
Among the given reactions, the reaction closest to having a ΔS (change in entropy) of zero at 25 degrees Celsius is reaction B: H2 (g) + I2 (s) -> 2HI (g).
Entropy is a measure of the disorder or randomness of a system. A reaction with a ΔS value close to zero indicates minimal change in entropy. To determine the relative ΔS values for the reactions, we consider the following factors:
A) C2H4 (g) + Br2 (l) -> C2H4Br2 (l)
This reaction involves the formation of a liquid from a gas and a liquid, which generally increases entropy.
C) N2 (g) + O2 (g) -> 2NO (g)
This reaction involves the formation of gas molecules from gas molecules, resulting in an increase in entropy.
D) CH3CHO (g) + 5/2O2 (g) -> 2CO2 (g) + 2H2O (g)
This reaction involves the formation of gas molecules from gas molecules, resulting in an increase in entropy.
E) 2NO (g) + O2 (g) -> 2NO2 (g)
This reaction involves the formation of gas molecules from gas molecules, resulting in an increase in entropy.
B) H2 (g) + I2 (s) -> 2HI (g)
In this reaction, a solid reacts with a gas to form a gas, resulting in a decrease in entropy.
Since reaction B involves a decrease in entropy, it is the closest to having a ΔS value of zero at 25 degrees Celsius.
Learn more about entropy here: brainly.com/question/20166134
#SPJ11
How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M?
Answers
Answer:
I think you mean 0.025 M and this is the answer
Explanation:
If a chemical reaction has a negative AH and a negative AS, then A) it will be spontaneous at all temperatures. B) it will be non-spontaneous at all temperatures. C) it will be spontaneous at high temperatures and non-spontaneous at low temperatures. D) it will be spontaneous at low temperatures and non-spontaneous at high temperatures. E) the absolute entropy of the products will be less than 0
Answers
If a chemical reaction has a negative AH and a negative AS, then it will be spontaneous at low temperatures and non-spontaneous at high temperatures.
The spontaneity of a reaction is determined by the sign and magnitude of the change in Gibbs free energy (G). The equation for calculating Gibbs free energy is:
ΔG = ΔH - TΔS
where H is the change in enthalpy, T is the temperature in kelvins, and S is the change in entropy.
When G is negative, the reaction is spontaneous.
When G is positive, the reaction is non-spontaneous.
When G is zero, the reaction is at equilibrium. If a chemical reaction has a negative H and a negative S, then the formula for Gibbs free energy becomes:
G = H - TS = (-) - T(-) When T is low, -TS will be small, so G will be negative, and the reaction will be spontaneous.
When T is high, -TS will be large, so G will be positive and the reaction will be non-spontaneous.
Therefore, the answer is D
It will be spontaneous at low temperatures and non-spontaneous at high temperatures.
To know more about the spontaneous reaction https://brainly.com/question/31199175
#SPJ11
Which phrase most accurately defines physiology? A. The study of how parts of the body function and work together B. The study of the structure of living things C The study one does before becoming a doctor
Answers
Answer:
a
Explanation:
The phrase most accurately defines physiology is option A the study of how parts of the body function and work together.
What is physiology ?The word "physiology" means "the scientific investigation of the mechanics and operations of a living system. Physiology is a branch of biology that focuses on how animals, organ systems, specific organs, cells, and biomolecules perform the chemical and physical processes necessary for a living system to operate.
The discipline can be separated into medical physiology, animal physiology, plant physiology, cell physiology, and comparative physiology in accordance with the types of organisms.
Physiological changes can have an effect on how people think. Examples of this include the results of specific drugs or the presence of dangerous amounts of chemicals.
Find more on physiology:
https://brainly.com/question/13208277
#SPJ6
please help, ive been putting 3 for the coefficient and 14 for the exponent but, it keeps saying it’s wrong. any help is appreciated.

Answers
Answer:
3 × 10¯¹⁰
Explanation:
9×10² ÷ 3×10¹²
The above expression can be evaluated as follow:
9×10² ÷ 3×10¹²
Recall:
3² = 9
3¹ = 3
Therefore,
9×10² ÷ 3×10¹² = 3²×10² ÷ 3¹×10¹²
Recall:
y^m ÷ y^n = y^(m – n)
Therefore,
3²×10² ÷ 3×10¹² = 3²¯¹ × 10²¯¹²
= 3¹ × 10¯¹⁰
Recall:
y¹ = y
Therefore,
3¹ × 10¯¹⁰ = 3 × 10¯¹⁰
Therefore,
9×10² ÷ 3×10¹² = 3 × 10¯¹⁰
The oxides of ______ are commonly used as oxidizing agents in organic synthesis.
Answers
The oxides of Cr(VI) and Mn(VII) are commonly used as oxidizing agents in organic synthesis.
What are oxidizing agents?An oxidizing agent is a substance that gains or accepts or receives" an electron from a reducing agent in a redox chemical reaction.
In other words, an oxidizer is any substance that oxidizes another substance.
Fluorine (F) is the most powerful oxidizing agent of all elements, and the other Halogens are also effective oxidizers.
Thus, in organic synthesis, the oxides of Cr(VI) and Mn(VII) are commonly used as oxidizing agents.
For more details regarding oxidizing agents, visit:
https://brainly.com/question/10547418
#SPJ4
3) Which of the following atoms has the smallest number of neutrons?
A) nitrogen-14
®) carbon-14
C) oxygen-16
D) neon-20
and third ene
Answers
Answer:
carbon-14..............
The atom which has the smallest number of neutrons is carbon-14 as it has the least atomic number.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ6
Is MgS ionic or covalent
Answers
Answer:
Ionic
Explanation:
Mg has 2 electron in it's outermost orbit, by donating this two electron to Sulfur it get stable mg 2+ electronic configuration while sulfur has 6 electron in it's outermost shell ,so sulfur accept this 2 electron and complete it's octet and become s2–.