Please help ill give brainiest
red tape can be used to repair a broken taillight a car. In one or two sentences, explain how different colors of light are
transmitted, reflected, and absorbed by this kind of tape. (2 points)
Answers
Red tape can be used to repair a broken taillight on a car. Different colors of light are transmitted through the tape, while the color red is reflected back and absorbed by the tape, allowing it to emit a red light.
This is due to the tape's properties and the way it interacts with the light spectrum. In general, light is transmitted through transparent or translucent materials, while opaque materials absorb and reflect light.
The color of an object is determined by the wavelengths of light that are absorbed and reflected by its surface. So, in the case of the red tape, it absorbs all colors of light except for red, which it reflects back, allowing the tape to emit a red light when placed over a broken taillight.
To know more about broken taillight refer here
https://brainly.com/question/16647309#
#SPJ11
Related Questions
what does it mean to say that an enzyme-catalyzed reaction is either enzyme-limited or substrate-limited?
Answers
Nothing changes; it stays the same.
What does it imply to claim that a reaction is being catalysed by an enzyme?
Enzymatic catalysis of a reaction involving two substrates. The two substrates are brought together in the correct direction and location to react with one another using the template provided by the enzyme.
How do enzymes decide what to eat for fuel?
Finding the peptide sequences that proteases cleave in vitro—or, more specifically, which amino acids span the cleavage site and are recognised by the enzyme's active site—is one method of identifying prospective protease substrates. The proteome is then searched for substrates using these sequences, much like partial licence plate numbers
To know more about Enzyme
Visit:
https://brainly.com/question/1996362
#SPJ4
HURRYY!!! WORTH 25pts. How many moles of a gas will be needed to fill a 42 liter container at 370 K and 9.16 atmospheres?
PV=nRT
R = 0.0821
Answers
Answer:
12.665 mols
Explanation:
You can just plug into the equation finding n, which is moles. The equation would be n = PV/RT. atm is 9.16, Volume is 42, 0.08206 or 0.0821 in this case is the constant, and T is the temperature which is 370 Kelvin.
Determine the equilibrium constant for the following reaction at 298 K. CIO(g) + O2(g) → Cl(g) + O3(8) AG° = 34.5 kJ/mol 0.986 4.98 x 10-4 8.96 x 10-7 5.66 x 105 1.12 x 106
Answers
the equilibrium constant for the given reaction at 298 K is 8.96 x 10^-7.
The equilibrium constant for the given reaction, CIO(g) + O2(g) → Cl(g) + O3(g), at 298 K can be determined using the Gibbs free energy of the reaction and the following equation:ΔG° = - RT lnK
where ΔG° is the standard Gibbs free energy change, R is the gas constant, T is the temperature in Kelvin, and K is the equilibrium constant.
The equation can be rearranged to solve for K:K = e^(-ΔG°/RT)where e is the natural logarithmic base, and all other variables are the same as in the previous equation.Substituting the given values,
we have:ΔG° = 34.5 kJ/molR = 8.314 J/(mol·K)T = 298 K
Using these values, we get:-
ΔG°/RT = (-34.5 × 10^3 J/mol) / (8.314 J/(mol·K) × 298 K)
= -13.19e^(-ΔG°/RT) = e^(-13.19) = 8.96 × 10^-7
Therefore, the equilibrium constant for the given reaction at 298 K is 8.96 x 10^-7.
learn more about equilibrium constant here
https://brainly.com/question/3159758
#SPJ11
Scientists may design an experiment with a control group, which is a set of organisms or sam-ples that do NOT receive the treatment (the independent variable) that is being tested. Scientists can then compare normal changes in organisms or samples with those that might have occurred because of the treatment. The idea of a "control group" is not the same as a "controlled variable." Suppose a scientist is doing an experiment to determine the effect of a cancer drug on mice with lymphoma
Answers
Answer:
See explanation
Explanation:
I believe that the aim of the scientist is to determine the effect of a cancer drug on mice with lymphoma. In this experiment, the mice with lymphoma are exposed to the drug. This is the treatment in the experiment. A control group of mice having lymphoma is not exposed to this treatment, this is the control group. This control group establishes a baseline for the study.
By comparing the outcome of the experimental and control groups, the effect of a cancer drug on mice with lymphoma can be determined.
How many electrons do carbon and oxygen share?
I-0-I
:0:
Intro
Done
Answers
oxygen has 6 valence electrons and 2 electrons and carbon has 4 electron
Answer:
4!!!!!!!!!!
Explanation:
i got it right
What would be the answer for these?

Answers
1. 4
2. 4
3. 2
4. 2
Further explanationGiven
Reaction
2H₂+O₂⇒2H₂O
Required
Number of atoms
Solution
In compounds, the number of atoms of the constituent components in the form of elements is usually indicated by a subscript after the element symbol, while the number in front of the compound which is the coefficient indicates the number of molecules of the compound.
In chemical reactions, the reactants are on the left and the products on the right
1. Number of Hydrogen in reactants
2 molecules x 2 atoms = 4 atoms
2. Number of Hydrogen in products
2 molecules x 2 atoms = 4 atoms
3. Number of Oxygen in reactants
1 molecules x 2 atoms = 2 atoms
3. Number of Oxygen in products
2 molecules x 1 atoms = 2 atoms
The reaction is said to be balanced because the number of atoms of the element is the same between the reactants and products
which of the following statements correctly describe the relative stabilities of resonance forms? select all that apply. multiple select question. a resonance structure with multiple bonds is always preferred to a resonance structure with only single bonds. a resonance structure with like charges on adjacent atoms is more stable than one with opposite charges on adjacent atoms. a resonance structure with formal charges closer to zero on individual atoms is preferred. a resonance structure is more stable if a negative charge resides on a more electronegative atom.
Answers
A resonance structure with formal charges closer to zero on individual atoms is preferred and resonance structure is more stable if a negative charge resides on a more electronegative atom.
Chemistry concept known as the theory of resonance that postulates that a molecule's actual normal state is represented by a combination of several alternative distinct structures rather than by a single valence-bond structure. The molecule is then said to have a structure that is a resonance hybrid of these structures or to resonate among the various valence-bond structures. The molecule is said to be stabilized by resonance when the calculated energy for a resonance hybrid is lower than the energies of any of the alternative structures. Resonance energy is the difference between the energies of any alternative structure and the energies of the resonance hybrid.
To know more about resonance visit :https://brainly.com/question/12087801
#SPJ4
349+1.10 + 100 =
and i have to put it in sig fig?
Answers
why water has more than one atom?
Answers
Answer:
In general, electrons can be shared between atoms (a molecular bond) or electrons can be completely removed from one atom and given to another (an ionic bond). ... Water is a molecule because it contains molecular bonds. Water is also a compound because it is made from more than one kind of element (oxygen and hydrogen).
Explanation:
7. Ionic solids use regular, repeating patterns to create crystal-like structures.
Fe+2 and S-2 are shown below. Use 10 cations and 10 anions to build the crystal
lattice structure of FeS (your final model should have 20 circles.)
Answers
Ionic solids use regular, repeating patterns to create crystal-like structures. Therefore, lattice structure of FeS is of cubic.
What is Ionic solids?Ionic solids would those be crystalline solids that include ions with opposite charges that are kept together by intense electrostatic forces of attraction. (ionic bonds). Examples: Ionic solids like NaCl, KBr, etc. have crystals. Describe how the anions and cations are arranged using a clear geometric design.
Describe how the anions and cations are arranged using a clear geometric design. They thus exist at room temperature as crystalline solids. They never exist as liquids or gases under normal pressure and temperature circumstances. Lattice structure of FeS is of cubic.
Therefore, lattice structure of FeS is of cubic.
To learn more about Ionic solids, here:
https://brainly.com/question/15241125
#SPJ1
A block that has a mass of 24 grams is 3 cm high, 4 cm long, and 7 cm wide. What is the density of this block?
Answers
A block that has a mass of 24 grams is 3 cm high, 4 cm long, and 7 cm wide. Therefore, the density is 0.28g/cm³.
What is density?Density is the mass of a specific material per unit volume. Density is defined as d = M/V, in which d represents density, M is weight, as well as V is volume. Density is generally expressed in grams every cubic centimetre. Water, for example, has a density of 1 gram every cubic centimetre, but Earth has a density of 5.51 grams every cubic centimetre.
Density is sometimes measured in kilos every cubic centimeter (in metre-kilogram-second or SI units). The density of air, for example, is 1.2 kilograms for every cubic metre. Textbooks and handbooks list the densities of various solids, liquids, including gases.
density= mass /volume
mass = 24 grams
volume= 3×4×7=84cm³
density= 24 /84=0.28g/cm³
Therefore, the density is 0.28g/cm³.
To learn more about density, here:
https://brainly.com/question/29775886
#SPJ1
skeletal structure for 5-isopentyldecane
Answers
Isopentane, commonly known as methyl butane or 2-methylbutane, is an alkane with a branched chain and five carbon atoms. Its chemical formula is C 5H 12 or CH(CH).
At normal temperature and pressure, isopentane is a highly flammable and volatile liquid. Additionally, under normal circumstances, it is the least dense liquid. [Reference needed] Isopentane has a standard boiling point that is only a few degrees above room temperature, therefore on a warm day, it will easily boil and evaporate. To achieve a liquid bath temperature of 160 °C, isopentane and liquid nitrogen are frequently utilized. Isopentane is a substantial component of natural gasoline, however, it normally only makes up 1% or less of natural gas.
To find more on Isopentane refer here:
https://brainly.com/question/5138367
#SPJ4
Given this equation: N2 + 3 H2 → 2 NH3, how many moles of NH3 can be produced from 3.1 moles of H2?
Answers
First, we write down our reaction:
N2 + 3H2 → 2NH3
Don't forget to balance it.
We only use moles as units.
Procedure:
3 x 1 mole H2 ------------ 2 x 1 mole NH3
3.1 moles H2 ------------- x
x = 2.1 moles NH3 are produced
Answer: 2.1 moles NH3
Is oxygen and potassium an ionic bond?
Answers
Ionic bond: Metal & Nonmetal
Brainliest is appreciated
Somebody pls help me with this question thx

Answers
Answer:
cell wall > skeletal system (maybe)
cell membrane > skin (maybe)
nucleus > brain
mitochondria > digestive system
Explanation:
Which two words apply to the substance copper sulphate?
Please give 1 answer.
A.
solid, compound
В.
gas, element
C
solid, mixture
D.
gas, compound
Answers
Answer: A. solid, compound
Explanation:
Because copper sulphate is a compound and it is a blue solid powder
Hope that help :)
What the anode , cathode and the electrolyte of a cell tha t you might use to electrolyte a spoon made from iron with silver?
Answers
The silver coating on the spoon is produced. When electrolyzing a spoon made from iron with silver, the anode, cathode, and electrolyte that can be used are as follows:
Anode: The anode is a negatively charged electrode, usually made of metal or graphite, that releases electrons during electrolysis. It is made of pure silver.Cathode: The cathode is a positively charged electrode that receives electrons during electrolysis. It is made of iron.Electrolyte: The electrolyte is a solution that conducts electricity and contains ions that can be reduced or oxidized. The electrolyte used for this process is a solution of silver nitrate (AgNO3) in water.The silver ion (Ag+) moves from the anode to the cathode through the electrolyte. At the cathode, it accepts an electron, reducing it to metallic silver (Ag). Fe(s) is oxidized to Fe2+(aq) ion at the anode, while Ag+ ions are reduced to Ag(s) at the cathode. Therefore, the silver coating on the spoon is produced.For such more questions on silver coating
https://brainly.com/question/29736740
#SPJ8
Elements that readily lose electrons tend to have
A) high ionization energy and high electronegativity
B) high ionization energy and low electronegativity
C) low ionization energy and low electronegativity
D) low ionization energy and high electronegativity
Answers
Answer:
C) low ionization energy and low electronegativity
Explanation:
which of the following are direct and indirect sources of particulate matter quarrying activities, farming activities, coal powered stations, factories
Answers
Coal powered stations and factories are direct and indirect sources of particulate matter.
One of the worst types of pollution in the air in India and around the world is particle pollution, also known as particulate matter pollution. Human activities are the main source of the increase in particle pollution, a type of air pollution.
Factories, power plants, incinerators, industries, autos, and diesel generators are major contributors of particulate matter emissions. All of this has human origins or is the result of human activity. Coal powered stations and factories are direct and indirect sources of particulate matter.
To know more about particulate matter, here:
https://brainly.com/question/15230454
#SPJ1
What is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL?
Answers
2gcm³ is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL.
What is density ?The term density is define as the ratio of mass and volume. The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimeter.
Density = mass / volume
Given:
Density = ?
Mass = 1.5 gram
Volume = 0.75 ml
By substituting this values in give equation we get,
Density = 1.5 / 0.75
= 2 gcm³
Thus, 2 gcm³ is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL.
To learn more about the density, follow the link;
https://brainly.com/question/29775886
#SPJ1
gas of unknown molecular mass was allowed to effuse through a small opening under constant pressure conditions. it required 57 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 26 s for 1.0 l of o2 gas to effuse. calculate the molar mass of the unknown gas.
Answers
The molar mass of the other gas is 153.79 g/mol
Step 1: The Data given,
It required 57 s for 1.0 L of the gas to effuse.
Under identical experimental conditions it required 26 s for 1.0 L of O2 gas to effuse.
Step 2: Calculate the molar mass
Graham's law of effusion says:
r1/r2 = √(M2/M1)
⇒ with r1 = effusion rate 1 = L/57s
⇒ with r2 = effusion rate 2 = L/26s
⇒ with M1 = molar mass of the gas = x g/mol
⇒ with M2 = molar mass of oxygen = 32 g/mol
We should be careful if we plug in the effusion rate. Because the fractions could flip if we don't give the effusion rates as numeral values.
(L/57s)/(L/26s) = 26/57 = √(32/M1)
26/57 =√(32/X)
(26/57) ² = 32/X
0.208064 =32/X
X = 32/0.208064
X = 153.79 g/mol
Hence, The molar mass of the other gas is 153.79 g/mol
To know more about Molar Mass -
https://brainly.com/question/12127540
#SPJ4
which is not a correct ion?
li2+
cl-
Al3
O2-
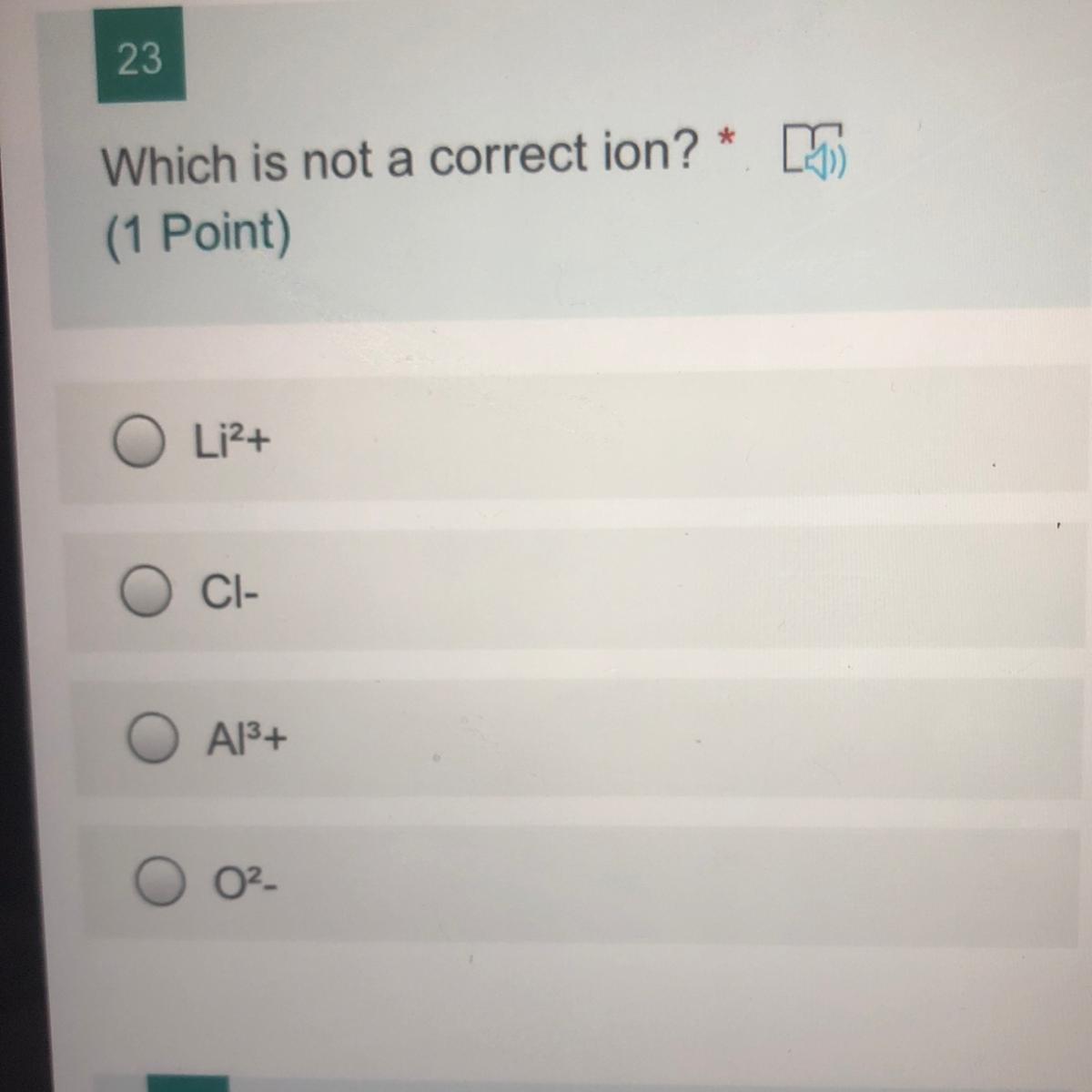
Answers
Answer:
The answer is option 1.
Explanation:
Lithium is a Group 1 element. So the correct charge for lithium should be Li+.
Answer:
(a)\(Li^{2+}\)
Explanation:
Answer is (a)\(Li^{2+}\)
As lithium has only one valence electron, it will lose one electron to form a cation with charge 1+ . Thus the correct Li ion is \(Li^{1+}\)
2
How many molecules are in 2.47 moles of CC14? *
Answers
1.49x10^24 molecules
describe how an ion is made
Answers
EXPLANATION- saw on the internet
Find the concentration of a solution that is prepared by mixing 15 g of sugar
and 125 ml of water.
Answers
Answer: Use the formula x = (c ÷ V) × 100 to convert the concentration (c) and volume (V) of the final solution to a percentage. In the example, c = 60 ml and V = 350 ml.
Explanation: Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.
Given the name of an element and the number of neutrons, find the mass of an Isotope

Answers
The given element nitrogen have 7 electrons and 7 protons. Mass number is the sum of number of protons and neutron. The number of neutrons is 7. Thus mass number of the isotope is 14.
What is nitrogen?Nitrogen is 7th element in periodic table. It is an electronegative element and is included in p block. Nitrogen exists as gas but sometime it can be liquified under high pressure.
Isotopes are atoms with same atomic number and different mass number. Nitrogen have two isotopes. N-14 and N-15. Mass number is the sum of number of protons and neutrons.
Even though isotopes are different in mass number their chemical properties will be similar but there exists slight changes in their physical properties.
Here, we have 7 protons and 7 electrons therefore, the mass number 14.Thus the isotope given in the diagram is N-14.
To learn more about nitrogen, refer the link below:
https://brainly.com/question/16113371
#SPJ5
what do I do when my dog ate my secret 10 pound stash of chocolate and he won't move please help what do i do oh god help me pls
Answers
Answer:
call an vet ASAP 10 pounds of chocolate is very bad for a dog.
Explanation:
Chocolate is one of the worst things a dog can eat, and 10 pounds of it is worst! Take your dog to the vet IMMEDIATELY! I hope that that the dog will survive.
-------------------------------------------------------------------------------------------------------------Brainiest would make my day, but you don’t have to give it! You’re welcome.
has 2 valence electrons. It has electron shells and is reactive than Boron. This element has the same number of electron shells as has 4 valence electrons. It has electron shells and is reactive than Aluminum. This element has the same number of electron shells as
Answers
Answer:
Beryllium.
Silicon.
Explanation:
Beryllium has 2 electrons in their outermost shell and has highly reactive than boron because Boron needs 3 electrons to lose whereas, beryllium needs to lose only 2 electrons to get stability. Silicon has 4 electrons in its outermost shell and is less reactive than aluminum because aluminum has to lose 3 electrons to get stability as compared to silicon which needs 4 electrons to lose to be stable and non-reactive.
A compound composed of only carbon and chlorine is 85. 5 % chlorine by mass. Propose a lewis structure for the lightest of the possible compounds that allows each atom to have a complete octet without formal charges.
Answers
Dichlorocarbene CCl2 is this substance.
Utilize 100 grams of the substance:
ω(Cl) = 85.5% ÷ 100%.
The mass proportion of chlorine in the chemical is (Cl) = 0.855.
m(Cl) = 0.855 · 100 g.
Chlorine mass is 85.5 grams, or m(Cl).
m(C) = 100 g - 85.5 g.
Carbon mass is 14.5 grams, or m(C).
M = n(Cl) = m(Cl) (Cl).
85.5 g x 35.45 g/mol = n(Cl).
n(Cl) = 2.41 mol; chlorine content.
n(C) equals 14.5 g x 12 g/mol.
Carbon quantity, n(C), is 1.21 mol.
n(Cl) = 2.41 mol, n(C) = 1.21 mol, and 1 = 2.
Dichlorocarbene CCl2 is the chemical name for this compound. The reactive intermediate with the chemical formula CCl2 is dichlorocarbene. Despite not having been isolated, this chemical species is a typical intermediate in organic chemistry since it is produced from chloroform. This twisted diamagnetic molecule enters other bonds quickly.
To know more about this problem:
https://brainly.com/question/6683097
#SPJ4

Predict the polarity of 6 real molecules (O2, HF, H2O, NH3, CF4, CH3F). First, draw the
molecules and any bond dipoles. Then draw any molecular dipoles. Explain your
reasoning before you check your predictions with the simulation.
Answers
The polarity of 6 real molecules are given below,
O2- Neutral
HF- Acidic
H2O- Neutral
NH3- Basic
CF4-Nonpolar
CH3F- Polar
How to determine polarity of molecules?Predicting the polarity of molecules can be done using a variety of methods. One method is to use the molecular dipole moment, which is a measure of the separation of positive and negative charge in a molecule. Molecular dipole moments can be calculated using quantum chemistry methods such as Density Functional Theory, or other methods. Another method is to consider the electronegativity of the atoms in the molecule and the type of bond between them. Non-polar molecules have atoms with similar electronegativities and strong covalent bonds, while polar molecules have atoms with different electronegativities and polar covalent or ionic bonds. Finally, the polarity of a molecule can also be predicted by looking at its shape and symmetry. Non-polar molecules often have symmetrical shapes, while polar molecules tend to be asymmetrical.To learn more about polarity of molecules refer :
brainly.com/question/30329106
#SPJ1