Answers
In the main group, the nonmetals come before the metalloids and then the metals.
What is the order of elementsThe elements in the periodic table consists of metals, metalloids and non metals. The periodic table is arranged in groups and periods. The groups are vertical while the periods are horizontal.
As you move from top to bottom in the main group, you move from Nonmetals, metalloids, metals.
Learn more about metals: https://brainly.com/question/25090336
Answer:
A. nonmetals, mettalloids, metals
Explanation:
Related Questions
a compound has an empirical formula of NaO and a molar mass of 78g/mol. what is its molecular formula
Answers
Answer:
Na2O2
Explanation:
Molecular Formula= Molar Mass/Empirical formula mass
To find empirical formula it is atomic weight of each element times the subscript added together. so since no subscript just mass of the elements added together
Na=23
O=16
23+16=39
78/39 = 2
2 times each element and thats the subscript
Predict the most likely bond type for the following.
a. Cu (Copper)
b. KCl (Potassium Chloride)
c. Si (Silicon)
d. CdTe (Cadmium Telluride)
e. ZnTe (Zinc Telluride)
Answers
Answer:
The three major types of bond are ionic, polar covalent, and covalent bonds. Ionic occurs majorly between metals and non-metals, which allows sharing of electrons to form an ionic compound. Whereas covalent bonding calls for complete transfer of electrons between atoms. Polar covalent bonds have unequaly shared electron-pair between two atoms.
Explanation:
a. Cu (Copper)- ionic bonding
b. KCl (Potassium Chloride) - ionic bonding
c. Si (Silicon) - covalent bonding
d. CdTe (Cadmium Telluride) - polar covalent bonding
e. ZnTe (Zinc Telluride)- polar covalent bonding
Please help answer this Guy's HELP it's very Important

Answers
I cant really help you tight now sorry
A hydrogen atom absorbs a photon of visible light and its electron enters the n = 4 energy level. Calculate the change in energy of the atom and the wavelength (nm) of the photon.
Answers
11. A covalent bond between C and H is_____. It is called that,because electrons are shared_____ between these two atoms.C and H,are not________
12. An______ bond is formed when an electron is transferred from one atom to another.
The atom that loses an electron is called a(n)________and has
charge________.The
atom that gains an electron is called a(n)__________and has_______charge.
13. A hydrogen bond is formed between a H in one molecule and an________atom in
another molecule. The H can make an H-bond only if it is found in a_________covalent
bond, in its own molecule.
14. H-bonds give________
many of its special properties.
15. A van der Waals interaction is the__________type of bond. All molecules form these
bonds. These interactions become important when found in great numbers. The often help the
3D shape of large molecules such as________Or________.
Answers
11.) Non polar, equally, Charged.
12.) Ionic, Cation, Positive, anion, negative
13.) electronegative, Polar
14.) So
15. weak, HCl or N2
A covalent bond between C and H is Non Polar. It is called that,because electrons are shared Equally between these two atoms.C and H,are not Charged
12. An Ionic bond is formed when an electron is transferred from one atom to another.
The atom that loses an electron is called Cation and has charge positive. The
atom that gains an electron is called anion and has negative charge.
13. A hydrogen bond is formed between a H in one molecule and an Electronegative atom in
another molecule. The H can make an H-bond only if it is found in a Polar covalent
bond, in its own molecule.
H-bonds give So many of its special properties.
A van der Waals interaction is the Weak type of bond. All molecules form these
bonds. These interactions become important when found in great numbers. The often help the
3D shape of large molecules such as HCL or N2
Learn more about covalent bond here:
https://brainly.com/question/3447218
#SPJ9
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
How many moles of Cr are in 1.0 mol of K2Cr2O7?
Answers
The molecular mass of \(K_{2}Cr_{2}O_{7}\) is \(294.1846g/mol\). \(Cr\) will have \(1 mole\)
This compound is known as potassium dichromate. Convert \(K_{2}Cr_{2}O_{7}\) between grams and moles or vice versa.
What do you mean by molesThe quantity (\(10\)) of material having \(6.023*10^{23}\) particles is known as a mole. \(6.023*10^{23}\) elements make up one mole. Avogadro's number is another name for this integer. We should be aware that the meaning of a mole is a quantity of a substance.
In the given question,
The chemical formula of potassium dichromate is K2Cr2O7, which indicates that it contains 2 moles of potassium (K), 2 moles of chromium (Cr), and 7 moles of oxygen (O).
Therefore, in 1.0 mol of K2Cr2O7, there are 2 moles of chromium (Cr).
To learn more about moles
brainly.com/question/26416088
#SPJ9
A 75.0- mL
volume of 0.200 M
NH3
( Kb=1.8×10−5
) is titrated with 0.500 M
HNO3
. Calculate the pH
after the addition of 17.0 mL
of HNO3
.
Answers
Answer:
ok, here is your answer
Explanation:
i am going to solve this problem by using the ICE table method which is an easy method to determine the pH of a weak base with the given data of the problem.Given:Initial volume of NH3 solution (Vi) = 75.0 mLInitial concentration of NH3 solution (Ci) = 0.200 MInitial moles of NH3 solution (Ni) = Ci x Vi = 0.200 M x 75.0 mL = 0.0150 molesKb = 1.8 x 10^-5Moles of HNO3 added (n) = 0.500 M x 17.0 mL = 0.00850 molesVolume of NH3 solution after the addition of HNO3 (Vf) = 75.0 mL + 17.0 mL = 92.0 mLConcentration of NH3 solution after the addition of HNO3 (Cf) = Ni / Vf = 0.0150 moles / 92.0 mL = 0.163 MTo find the pH after the addition of 17.0 mL of HNO3, we need to use the ICE table method.ICE table method:Initial: NH3 + H2O ⇌ NH4+ + OH-Change: -x 0 +x +xEquilibrium: 0.0150 - x 0 x xKb = [NH4+][OH-] / [NH3]1.8 x 10^-5 = x^2 / 0.163Solving for x, x = 0.00171 M[OH-] = 0.00171 M[OH-] = Kw / [H3O+] = 1.0 x 10^-14 / [H3O+][H3O+] = 5.85 x 10^-12pH = -log[H3O+]pH = -log(5.85 x 10^-12)pH = 11.23Therefore, the pH after the addition of 17.0 mL of HNO3 is approximately 11.23.
mark me as brainliestUsing this balanced equation:
NaHCO3 + CH3COOH H2O + CO2 + NaC2H3O2
In an experiment , the following mass measured 3.0 grams, 5.5 grams and 7.0 grams of
sodium bicarbonate is mixed with 0.5 mol of acetic acid (vinegar) to form carbon dioxide as
a product formed
Answers
The stoichiometric ratio of NaHCO₃ to CH₃COOH, which is determined by the balancing equation, is 1:1, meaning that 1 mole of NaHCO₃ reacts with 1 mole of CH₃COOH.
What is the balanced equation between vinegar's acetic acid CH₃COOH and sodium bicarbonate NaHCO₃?Acetic acid (CH₃COOH), a component of vinegar, interacts with sodium bicarbonate (NaHCO₃) in baking soda to produce water, carbon dioxide gas, and sodium acetate.
When NaHCO₃ and HC₂H₃O₂ are joined in a closed system, what do you suppose would happen?CO₂(g) + H₂O(l) + NaC₂H₃O₂ = NaHCO₃(aq) + HC₂H₃O₂(aq) when yellow bubbles appear, an acidic gas H₂CO₃ has generated as a result of the reaction between CO₂ and water. The salt in the solution (NaC₂H₃O₂) is basic since the solution is red in colour.
To know more about stoichiometric visit:-
https://brainly.com/question/29856106
#SPJ1
A compound with a molecular mass of 44.0 grams is found to be 81.82% carbon
and 18.18% hydrogen by mass. Finds its molecular formula. (HINT: Once you get
the mole to mole ratio you will need to multiple both by 3)
Answers
The molecular formula of the compound containing 81.82% carbon
and 18.18% hydrogen by mass is C₃H₈
From the question given above, the following data were obtained:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Molar mass of compound = 44 g/mol
Molecular formula =?We'll begin by calculating the empirical formula of the compound. This can be obtained as follow:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Empirical formula =?Divide by their molar mass
C = 81.82 / 12 = 6.818
H = 18.18 / 1 = 18.18
Divide by the smallest
C = 6.818 / 6.818 = 1
H = 18.18 / 6.818 = 2.67
Multiply by 3 to express in whole number
C = 1 × 3 = 3
H = 2.67 × 3 = 8
Thus, the empirical formula of the compound is C₃H₈
Finally, we shall determine the molecular formula of the compound. This can be obtained as follow:
Molecular formula = Empirical formula × n = molar mass
[C₃H₈]n = 44
[(12×3) + (1×4)]n = 44
[36 + 4]n = 44
40n = 44
Divide both side by 40
n = 44/40
n ≈ 1
Molecular formula = C₃H₈ × n
Molecular formula = C₃H₈ × 1
Molecular formula = C₃H₈Therefore, the molecular formula of the compound is C₃H₈
Learn more: https://brainly.com/question/13208888
Read the passage and answer the question.
In 2000, when paleontologist Paul Sereno led an expedition into Ténéré desert in Niger looking for the fossils of dinosaurs and ancient crocodiles, the photographer Mike Hettwer wandered away from the group to take pictures of some dunes near the main dig site. The photographer quickly found bones sticking out of the dunes, but they were human bones, not the prehistoric reptile bones the group had been looking for. However, the group was not going to pass up such an amazing discovery.
During the site excavation, the paleontologists found dozens of gravesites. Some of them held skeletal remains and potsherds with wavy lines etched in them. The scientists named this group the Kiffians. The others held skeletal remains that indicated a taller group of people, and their potsherds were decorated with patterns of dots. The scientists named this group the Tenerians. The graves also contained tools and beads made from stones or bones, as well as refuse heaps containing the bones of the animals the people living in the area had consumed. Some of the graves didn't contain any pottery so it was a mystery which group they belonged to. It was also not known when these people lived in the desert or how they survived.
The carbon-14 dating revealed that the Kiffians lived in the area around 9,700 years ago, and then the area was abandoned until the Tenerians lived in the area 7,000 years ago. The scientists want to reconstruct what the land looked like when it was inhabited, as well as understand why there was about a 2,000-year-gap during which nobody lived in the area.
The scientists hypothesize that the two civilizations lived around a lake that dried up during periods of drought and then eventually reformed. The changing lake led people to move, depending on if they had a water source or not.
How can scientists use radiometric dating to reconstruct the geologic history of the area to support or reject their hypothesis of a disappearing and reappearing lake? What is a tool that scientists can use to ensure that their radiometric dating is accurate?
Answers
Answer & Explanation:
Scientists can use radiometric dating to reconstruct the geologic history of the area and support or reject their hypothesis of a disappearing and reappearing lake by analyzing the sediments and other geological features present in the area. Radiometric dating, such as carbon-14 dating, can determine the age of the sediments, fossilized remains, and other materials. By analyzing the age and composition of these materials, scientists can track changes in the environment over time, including periods of drought or increased precipitation that could cause a lake to dry up or reform.
To ensure the accuracy of their radiometric dating, scientists can use calibration methods, such as cross-dating with other dating techniques like dendrochronology (tree-ring dating), or comparing their results with well-dated samples from similar or nearby environments. This can help validate the radiometric dating results and provide more confidence in the reconstructed geologic history and any conclusions drawn from it regarding the presence or absence of a lake in the area at different times.
you are on vacation with your family up in the mountains. your friend is on vacation near the equator by the ocean.Explain which climate will experience the most precipitation.
A.both climates will have the same amount of precipitation. B.the ocean climate will have less precipitation.C. the mountain climate will have more precipitation. D.the climate around the equator will have more precipitation(this question is for science)
Answers
Answer:
option d is right
Explanation:
More precipitation occurs near the equator because it is a tropical area. Mountains receive less than 15 inches of rain per year while the equator area receives a lot of rain. This is due to the very hot weather.
The climate around the equator will have more precipitation because it is very hot.
thats the answer
If the Sun suddenly lost all of its gravity, which statement would best describe how the planets would move?
a
The planets would stop moving.
b
The planets would all collide into each other.
c
The planets would travel in a straight line.
d
The planets would continue moving in a circular path.
Answers
Answer:
It's B
Explanation:
It's the sun's gravitational pull that keeps our planets revolving around it and as soon as it's gone it's a high chance of it being a pinball machine and everything colliding into each other.
FAST PLSSS!!
It appears as though the sun disappears and the moon comes out at nighttime. Based on what you know, explain why this is happening at nighttime.
a
Earth is rotating towards the sun.
b
Earth is rotating away from the sun.
c
The moon is rotating toward the sun.
d
The moon is rotating away from the sun.
Answers
chemical reaction NaCl (aq) + AgNO 3 (aq) → AgCl (s) + NaNO 3 (aq) is:
Answers
Answer:
This reaction is both precipitation reaction as well as double displacement reaction.
In a double displacement reaction the anions are exchanged and in a precipitation reaction, a precipitate is formed in the end. In this case, AgCl is the precipitate.
1. A 25 g rock is placed in a graduated cylinder with water. The volume of the liquid rises from 18.3 mL to 21.4 mL Calculate the density of the rock in g/cm^3.
2. The density of aluminum is 2.7 g/mL If the length and width of a 1.08 kg. Al bar are 5 cm x 8 cm, then what is the height of the Al bar?
Answers
Answer:
1. \(\rho=8.06g/cm^3\)
2. \(H=10cm\)
Explanation:
Hello,
1. In this case, since the volume of the rock is obtained via the difference between the volume of the cylinder with the water and the rock and the volume of the cylinder with the water only:
\(V=21.4mL-18.3mL=3.1mL\)
Thus, the density turns out:
\(\rho=\frac{m}{V}=\frac{25g}{3.1cm^3} \\\\\rho=8.06g/cm^3\)
2. In this case, given the density and mass of aluminum we can compute its volume as follows:
\(V=\frac{m}{\rho}=\frac{1080g}{2.7g/cm^3}=400cm^3\)
Moreover, as the volume is also defined in terms of width, height and length:
\(V=W*H*L\)
The height is computed to be:
\(H=\frac{V}{L*W}=\frac{400cm^3}{5cm*8cm}\\ \\H=10cm\)
Best regards.
An organic compound consist the following by mass 0.188g of carbon, 0.062 of H and 0.25g of O. If the vapor density of the compound is 16. Determine the molecular formula
Answers
The compound has the molecular formula CH₄O. H : O = 1 : 4 : 1 As a result, the empirical formula is CH₄O.
Equating empirical formula :The organic compound sample's mass is 0.5 g, or (0.188 + 0.062 + 0.25) The vapor density should not have a unit.
The organic compound has a molar mass = 16 × 2 g/mol
= 32 g/mol
In 1 mol of the compound:Moles of C atoms = (32 g) × (0.188/0.5) / (12 g/mol) = 1
Moles of H atoms = (32 g) × (0.062/0.5) / (1 g/mol) = 4
Moles of O atoms = (32 g) × (0.25/0.5) / (16 g/mol) = 1
Hence, molecular formula = CH₄O
Thus, the compound has the molecular formula CH₄O. H : O = 1 : 4 : 1 As a result, the empirical formula is CH₄O.
What are examples of organic compounds?Organic molecules contain covalently bound carbon and hydrogen as well as frequently additional elements. Benzoic acid, diethyl malonate, propanoic acid, butanoic acid, malonic acid, amines, heterocyclic compounds, and benzoic aldehyde are all examples of organic compounds. Organic substances include the carbohydrates, fats (lipids), proteins, and nucleic acids that make up the components of life. Organic molecules include petroleum and natural gas, which constitute the majority of fossil fuels.
Learn more about organic compounds :
brainly.com/question/5994723
#SPJ1
Hydrogen gas contracts at constant pressure from 1.00 L to 0.95 L. The initial
temperature is 20 °C. Find the final temperature of the gas?
Answers

How many grams of Ag₂S₂O₃ from when 125.0g AgBr reacts completely according to the reaction below?
2AgBr + Na₂S₂O₃ → Ag₂S₂O₃ + 2NaBr
Ag₂S₂O₃ : 327.74 g / mol
AgBr : 187.70 g / mol
______g? Ag₂S₂O₃
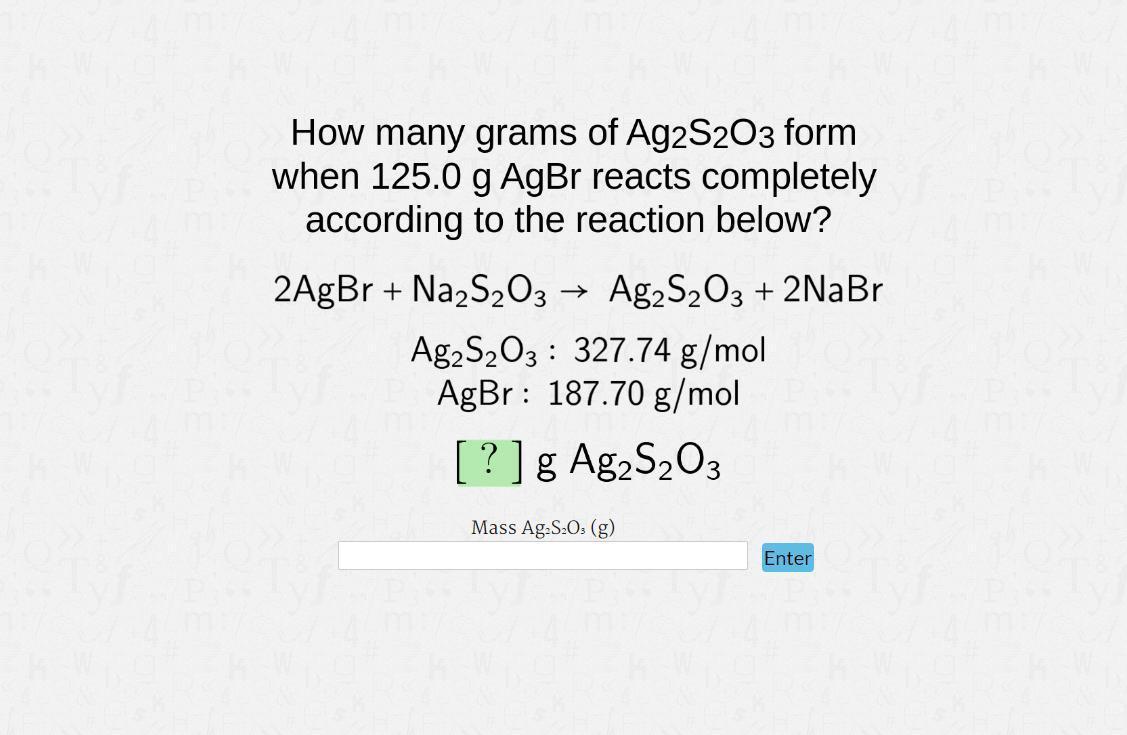
Answers
Since, 2AgBr + Na₂S₂O₃ → Ag₂S₂O₃ + 2NaBr
Ag₂S₂O₃= 327.74 g / mol
AgBr= 187.70 g / mol
Hence, amount of Ag₂S₂O₃ formed in this chemical reaction is 200g.
What is a chemical reaction?
Chemical reaction, a process in which one or more substances, the reactants, are transformed into one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the basic atoms of the reactants to form different substances as products.Chemical reactions must be distinguished from physical changes. Physical changes include changes of state, such as ice melting to water and water evaporating to steam. If a physical change occurs, the physical properties of the substance will change, but its chemical identity will remain the same. Regardless of its physical state, water (H2O) is the same compound, with each molecule consisting of two hydrogen atoms and one oxygen atom. However, when water, such as ice, liquid, or steam, meets metallic sodium (Na), the atoms rearrange to give the new substances molecular hydrogen (H2) and sodium hydroxide (NaOH).To know more about chemical reactions, click the link given below:
https://brainly.com/question/29039149
#SPJ4
You are making 100 ml of a 2 M solution of HCl. How many ml of a 4 M HCl solution will you require
Answers
Known :
M1 = 2 M
V1 = 100 mL
M2 = 4 M
Solution :
M1 • V1 = M2 • V2
(2 M)(100 mL) = (4 M)(V2)
V2 = 50 mL
Considering the definition of dilution, 50 mL of a 4 M HCl solution you will require.
What is dilutionWhen it is desired to prepare a less concentrated solution from a more concentrated one, it is called dilution.
Dilution is the process of reducing the concentration of solute in solution, which is accomplished by simply adding more solvent to the solution at the same amount of solute.
In a dilution the amount of solute does not change, but as more solvent is added, the concentration of the solute decreases, as the volume (and weight) of the solution increases.
A dilution is mathematically expressed as:
Ci×Vi = Cf×Vf
where
Ci: initial concentration.Vi: initial volume.Cf: final concentration.Vf: final volume.What is volume of a 4 M HCl solutionIn this case, you know:
Ci= 2 MVi= 100 mLCf= 4 MVf= ?Replacing in the definition of dilution:
2 M× 100 mL= 4 M× Vf
Solving:
Vf= (2 M× 100 mL)÷ 4 M
Vf= 50 mL
In summary, 50 mL of a 4 M HCl solution you will require.
Learn more about dilution:
brainly.com/question/20113402?referrer=searchResults
brainly.com/question/22762236?referrer=searchResults
#SPJ2
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Enter a balanced equation for the reaction of KOH and Cu(NO3)2 . Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no precipitate is formed.
Answers
For each compound, the states (aq) for aqueous, or dissolved in water, and (s) for solid, or precipitate created, are used to denote their states.
What occurs when sodium hydroxide interacts with copper II nitrate?A mixture made up of a sodium nitrate solution and a copper(II) hydroxide precipitate is created when copper(II) nitrate and sodium hydroxide solutions are combined.
KOH and Cu(NO3)2 react, and the balanced equation for the reaction is
2KOH(aq) + Cu(NO3)2(aq) Cu(OH)2(s) + 2KNO3 (aq)
Copper(II) hydroxide (Cu(OH)2) and potassium nitrate are produced in this reaction between potassium hydroxide (KOH) and copper(II) nitrate (Cu(NO3)2) (KNO3).
To know more about aqueous visit:-
https://brainly.com/question/30215562
#SPJ1
Which element has a greater electronegativity?
fluorine (9) or radium (88)
Answers
Answer:
Fluorine
Explanation:
Electronegativity increases as you go from left to right across the periodic table and decreases as you go from top to bottom of the periodic table.
Fluorine is in period 3, group 17
Radium is in period 7, group 2
Radium is in period 7 and we know that electronegativity decreases as you move from top to bottom.
Explanation: As you move from top to bottom, you are in higher energy level, which means that your distance from the nucleus is further away.
Answer:
Fluorine
General Formulas and Concepts:
Chemistry
Reading a Periodic TablePeriodic TrendsElectronegativity - the tendency for an element to attract an electron to itselfZ-effective and Coulomb's Law, Forces of AttractionExplanation:
The Periodic Trend for Electronegativity is up and to the right of the Periodic Table.
Fluorine is Element 9 and has 9 protons. Radium is Element 88 and has 88 protons. Therefore, Radium has a bigger Zeff than Flourine.
However, since Radium is in Period 7 while Fluorine is in Period 2, Radium has more core e⁻ than Fluorine does. This will create a much larger shielding effect, causing Radium's outermost e⁻ to have less FOA between them. Fluorine, since it has less core e⁻, the FOA between the nucleus and outershell e⁻ will be much stronger.
Therefore, Fluorine would attract an electron more than Radium, thus bringing us to the conclusion that Fluorine has a higher electronegativity.
Does the amount of air change the time it will take to burn completely?
Answers
Explanation:
The answer is yes. Adding O2 to a fire, feeds a fire creating bigger flames or almost completely burning a building,.. etc....
depending on what kind of structure.
little O2 usually makes a fire burn itself out because there is not enuf oxygen or its contained...
what is the frequency in herts of an xray having wavelength of 4.73 x 10-14 hectometers to 3 significant figures?
Answers
The frequency (in hertz) of the x-ray having a wavelength of 4.73×10⁻¹⁴ hectometers is 6.34×10¹⁹ Hertz
How do I determine the frequency of the x-ray?Frequency is defined as the number of complete oscillations made in 1 second.
However, the frequency of a wave is related to wavelength according to the following formula:
Velocity (v) = wavelength (λ) × frequency (f)
v = λf
With the above formula, we can determine the frequency of the x-ray. Details below:
Wavelength (λ) = 4.73×10⁻¹⁴ hectometers = 4.73×10⁻¹⁴ × 10² = 4.73×10⁻¹² metersSpeed of x-ray (v) = 3×10⁸ m/sFrequency (f) =?Velocity (v) = wavelength (λ) × frequency (f)
3×10⁸ = 4.73×10⁻¹² × frequency
Divide both sides by 4.73×10⁻¹²
Frequency = 3×10⁸ / 4.73×10⁻¹²
Frequency = 6.34×10¹⁹ Hertz
Thus, we can conclude, the frequency is 6.34×10¹⁹ Hertz
Learn more about frequency:
https://brainly.com/question/15326129
#SPJ1
An argon ion laser emits visible radiation with photons of energy 4.071 x 10-19 J. What is the
wavelength of the radiation?
Answers
The wavelength of the radiation emitted by the argon ion laser is \(4.854 * 10^-7 m\).
Wavelength is a property of any type of wave that refers to the distance between two adjacent points on the wave that is in phase, i.e., at the same point in their respective cycles. It is usually denoted by the Greek letter lambda (λ) and is measured in units of length, such as meters or nanometers.
The energy carried by the photon (E) is related to the wavelength (\(\lambda\)) through the following equation:
\(E=hc/\lambda\); where 'h' is the Plank's Constant and 'c' is the speed of light which is \(3* 10^{-7} m/s\).
We can say that
\(\lambda - hc/E\)
Now after substituting the given values, we get:
\(\lambda = (6.626 * 10^{-34} J.s * 3.00 * 10^8 m/s) / (4.071 * 10^{-19} J)\\\lambda = 4.854 * 10^-7 m\)
Therefore the wavelength of the radiation emitted by the argon ion laser is \(4.854 * 10^-7 m\).
Learn more about the Plank's Constant at:
https://brainly.com/question/28060145
#SPJ4
Please help me, im studying for finals and i need an answer to this question! Will mark brainliest for the best answers!w

Answers
Answer:
the generation of electricity and other energy jointly, especially the utilization of the steam left over from electricity generation to produce heat.
I hope it helps!! Have a nice day
Answer:
Cogeneration r is the use of a heat engine or power station to generate electricity and useful heat at the same time.
Research and describe one career in health and fitness that you would consider
Answers
Answer:
a PE teacher would be in both catagories of heath and fitness
Explanation:
The density of aluminium is 2.7 g/cm3. Find the mass in grams of a bar of aluminum measuring 1.7 cm by 3.0 cm by 12.9 cm.
Answers
Answer: 177.23 g.
Explanation:
the volume of the aluminum bar is
1.7 cm x 3.0 cm x 12.9 cm
= 65.61 cm^3
2.7 g/cm^3 x 65.61 cm^3
177.23g
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8