Answers
Answer:
aplha particle is the answer
Related Questions
Help a person out please
Answers
Explanation:
here I haven't drawn the diagram but I have marked them where they will be .
So if it helps don't forget to like and Mark me

Do covalent or ionic bonds have larger molecules
Answers
Answer:
No they not have larger Molecules .In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. The reaction components of covalent bonds are electrically neutral, whereas for ionic bonds they are both charged. Covalent bonds are formed between two non-metals, whereas ionic bonds are formed between a metal and non-metal.Hope this helps you !!what is the opposite of a heterotroph
Answers
The opposite of heterotroph is autotroph.
What is heterotroph?
An organism is referred to as a heterotroph if it consumes other plants or animals for food and energy. Its origins are in the Greek words hetero, which means "other," and trophe, which means "nutrition."
Examples - Human, birds, dogs, etc.
What is autotroph?
A primary producer, also known as an autotroph, is an organism that uses energy from light or inorganic chemical reactions to create complex organic chemicals from simple ones, such as carbon dioxide.
Example - plants, algae etc.
The opposite of heterotroph is autotroph.
To know more about autotroph, check out:
https://brainly.com/question/10253663
#SPJ1
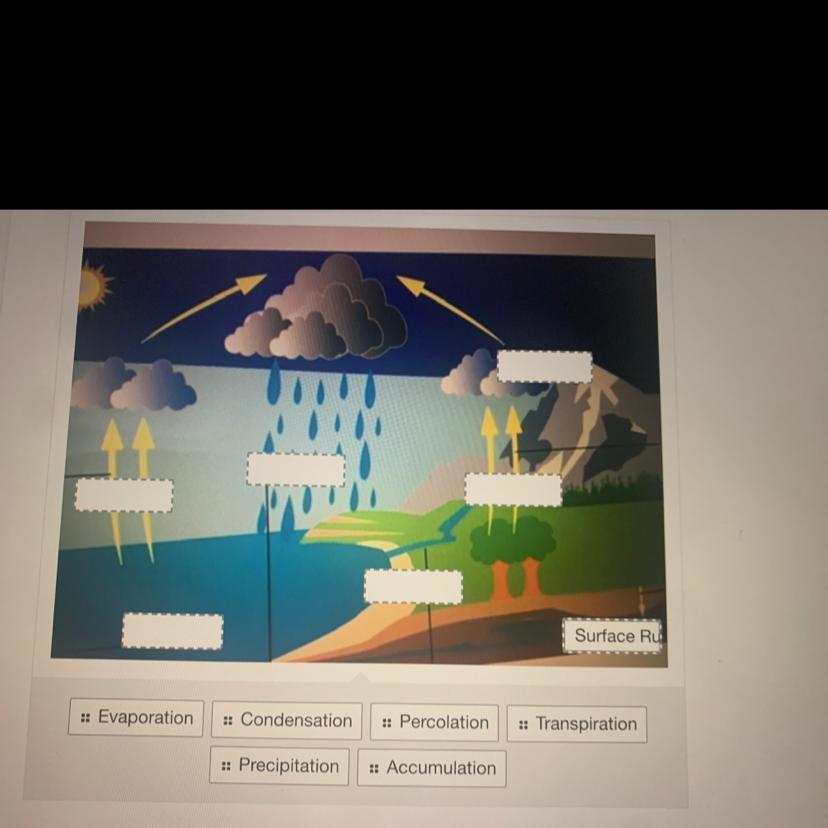
What happens to the ocean water after the condensation part of the water cycle?
The ocean water collects in lakes and streams. The ocean water turns into water vapor. The ocean water falls down as precipitation. The ocean water runs off Earth's surface
Answers
After the condensation part of the water cycle, the ocean water falls down as precipitation.
During the water cycle, ocean water first evaporates into the atmosphere due to the heat from the sun. This water vapor then cools and condenses to form clouds.
Once the clouds become heavy enough, the water falls back down to the Earth's surface as precipitation. This precipitation can then collect in lakes and streams or run off the Earth's surface, but it is important to note that these are steps that occur after the condensation part of the water cycle.
Therefore, the correct answer is that the ocean water falls down as precipitation after the condensation part of the water cycle.
Learn more about water cycle here: https://brainly.com/question/25796102.
#SPJ11
EASY POINTS GIVEN! WILL MARK BRAINLIEST:)

Answers
Answer:
faxs
Explanation:
Answer:
the first oneeeeeeeee
What is the final temperature of 15.6 g of aluminum that releases 277 J of heat, if the initial temp is 8.1 °C? (specific heat = 0.900 J/g°C).
Answers
If the initial temp is 8.1 °C and specific heat = 0.900 J/g°C, the final temperature of the aluminum is 10.2°C.
To solve this problem, we can use the formula Q = mcΔT, where Q is the amount of heat released, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature. We can rearrange this formula to solve for the final temperature, which is the quantity we are looking for.
First, we need to calculate the value of cΔT for the aluminum. We know that the mass of aluminum is 15.6 g, and the specific heat is 0.900 J/g°C. To find ΔT, we can use the formula ΔT = Q / (mc), where Q is the heat released, m is the mass of the aluminum, and c is the specific heat of aluminum.
Plugging in the values, we get:
ΔT = 277 J / (15.6 g * 0.900 J/g°C) = 20.0 °C
Next, we can use the formula Q = mcΔT again to solve for the final temperature. Plugging in the values we have, we get:
277 J = 15.6 g * 0.900 J/g°C * (T - 8.1°C + 20.0°C)
Simplifying this equation, we get:
277 J = 15.6 g * 0.900 J/g°C * (T + 11.9°C)
Solving for T, we get:
T = (277 J / (15.6 g * 0.900 J/g°C)) - 11.9°C = 10.2°C
To learn more about temperature click on,
https://brainly.com/question/28302912
#SPJ4
ohesion between water molecules contributes to __________ which allows the molecules to stick together and resist outside forces.
Answers
Answer: I believe the answer is surface tension :)
What do we call a substance composed of atoms of more than one element that are held together by chemical bonds?
Compound
Crystal
Salt
Ion
Answers
A substance composed of atoms of more than one element that are held together by chemical bonds is called a compound. Therefore the correct option is option A.
A compound is a pure material that is created by chemically combining two or more distinct components in a specific order. Chemical bonds, which can be ionic or covalent, hold the atoms of a substance together.
The characteristics of compounds are distinct from the characteristics of the constituent parts.
For instance, sodium is a soft metal and chlorine is a greenish-yellow gas; nevertheless, when these two elements combine to produce sodium chloride (table salt), they create a white crystalline solid that is far more stable than the constituent parts of each element alone. Therefore the correct option is option A.
For such more question on chemical bond:
https://brainly.com/question/819068
#SPJ11
Is Elisa getting the right amount of sleep??

Answers
Answer: Yes, because it’s between 8 and 10.
Explanation:
When this reaction is run , 57.75 g H2O is produced. What is the percent yield for this result?
Answers
The theoretical yield is the amount of product that would be obtained if the reaction proceeded with 100% efficiency.
Once you have the theoretical yield and the actual yield (which is given as 57.75 g of H2O in this case), you can use the following formula to calculate the percent yield:
Percent Yield = (Actual Yield / Theoretical Yield) x 100
In this case, the actual yield is 57.75 g and the theoretical yield is 60.00 g. Therefore, the percent yield is:
Percent yield = (57.75 g / 60.00 g) * 100% = 96.25%
Therefore, the percent yield for this reaction is 96.25%.
To know more about theoretical yield:
https://brainly.com/question/14966377
#SPJ1
chemical reactions differ from nuclear reactions in several important ways. match each description correctly to the type of reaction. atoms are rearranged by the breaking and forming of chemical bonds in a atoms are rearranged by the breaking and forming of chemical bonds in a drop zone empty. an element or isotope is converted into a different element or isotope in a an element or isotope is converted into a different element or isotope in a drop zone empty. the energy changes in a nuclear reaction are the energy changes in a nuclear reaction are drop zone empty. the energy changes in a chemical reaction are the energy changes in a chemical reaction are drop zone empty. nuclear reaction. relatively small. chemical reaction. extremely large.
Answers
Chemical reactions involve the rearrangement of atoms through breaking and forming chemical bonds, while nuclear reactions involve the conversion of an element or isotope into a different one.
Chemical reactions involve the breaking and forming of chemical bonds between atoms, resulting in the rearrangement of those atoms to create a new substance. These reactions typically involve relatively small energy changes.
On the other hand, nuclear reactions involve the conversion of one element or isotope into another, usually by emitting particles such as alpha or beta particles. The energy changes in nuclear reactions are typically extremely large compared to chemical reactions.
So, to summarize, chemical reactions involve rearrangement of atoms through breaking and forming of chemical bonds and have relatively small energy changes, while nuclear reactions involve conversion of one element or isotope to another and have extremely large energy changes.
Learn more about nuclear reactions here:
https://brainly.com/question/12786977
#SPJ11
A carboxylic acid does not form phenyl hydrozone when treated with phenyl hydrazine . explain
Answers
Due to the absence of a reactive carbonyl group, carboxylic acids do not form phenyl hydrazones when treated with phenyl hydrazine.
Phenyl hydrazone formation typically occurs when an aldehyde or ketone reacts with phenyl hydrazine. In this reaction, the carbonyl group (C=O) of the aldehyde or ketone undergoes a nucleophilic addition with the hydrazine group (-NHNH2) of phenyl hydrazine, resulting in the formation of a hydrazone.
However, carboxylic acids do not possess a reactive carbonyl group like aldehydes or ketones. Instead, carboxylic acids contain a carboxyl group (COOH) which is not susceptible to nucleophilic addition by phenyl hydrazine. The carboxylic acid group is more acidic in nature and does not participate in hydrazone formation.
Due to the absence of a reactive carbonyl group, carboxylic acids do not form phenyl hydrazones when treated with phenyl hydrazine. It is important to note that the reaction conditions and reagents should be carefully chosen to match the desired chemical transformation.
To learn more about carboxylic group, visit
https://brainly.com/question/13564853
#SPJ11
Living Directions: Classify the following type of potential energy (P) or kinetic energy (K).
6. Walking down the sidewalk.
7. A skier at the top of the mountain.
8. A pitcher throwing a baseball to first base.
9. Gasoline in a gas tank.
10. An archer with his bow drawn.
11. An apple on an apple tree in an orchard.
12. A car driving down the highway.
13. Water flowing down from a waterfall.
14. A soccer player kicking a soccer ball across the field.
Answers
7. Potential
8. Kinetic
9. Potential
10. Potential
11. Potential
12. Kinetic
13. Kinetic
14. Kinetic
**I think this is right, if not I’m sorry**
Good luck :D
Also, kinetic energy is *motion* and potential energy is when an object has the *potential* to move. Hope this helped! Have a great day :D
what bonded atom lone pair arrangement is predicted by vsepr theory for the electron groups that surround the carbon atom in co2?
a.Linear b.Trigonal planar
c. Bent d. Tetrahedral e.Trigonal pyramidal
Answers
According to the VSEPR theory, the electron group arrangement around the carbon atom in CO2 is indeed linear. However, you asked for the arrangement of bonded atom lone pairs. In the case of CO2, there are no lone pairs of electrons on the carbon atom.
CO2 has a linear molecular geometry, with the carbon atom at the center and two oxygen atoms bonded to it. Each oxygen atom forms a double bond with the carbon atom, and there are no additional lone pairs on the carbon atom. Therefore, the correct answer is (a) Linear, as there are no lone pairs of electrons around the carbon atom in CO2.
To know more about VSEPR theory refer here
https://brainly.com/question/7528668#
#SPJ11
draw the chemical reaction that occurs when the benzoic acid reacts with the naoh
Answers
The chemical reaction between benzoic acid (C₆H₅COOH) and NaOH can be represented as C₆H₅COOH + NaOH → C₆H₅COONa + H₂O.
This reaction involves the displacement of the hydrogen atom from the carboxylic acid group in benzoic acid by the sodium atom from sodium hydroxide. As a result, sodium benzoate (C₆H₅COONa) and water (H₂O) are formed.
The reaction between benzoic acid and NaOH is classified as an acid-base reaction, where the acidic benzoic acid reacts with the basic NaOH to produce a salt (sodium benzoate) and water.
To know more about benzoic acid click here:
https://brainly.com/question/3186444
#SPJ11
How did Mendeleev set up his Periodic Table of Elements?
Answers
URGENT HELP
26. A solution of hydrogen peroxide is 23.3% H2O2 by mass and has a density of 1.11
g/cm”. The molarity of the solution is:
a) 7.14 M
b) 0.259 M
c) 7.60 M
d) 7.93 M
e) none of these
Answers
34. When the equation
Fe2(SO4)3 + Ba(OH)2
is completed and balanced, one term in the balanced
equation is
(a) Ba2(SO4)3
(c) 2 Fe2(SO4)3
(b) 2 Fe(OH)2 (d) 2 Fe(OH)3
Answers
The balanced equation of the given reaction include the term 2Fe (OH)₃. Because, the Fe and OH- have to be balanced in both side. Hence, option d is correct.
What is balanced chemical equation ?The balanced chemical equation of a reaction represents the prefect stoichiometry of all the reactants and products. The number of each element in the reactant side much must be equal to their number in the product side.
Therefore, no atoms can be lost or produced extra in a reaction. Thus, the total mass become conserved for the system. The complete balanced reaction between iron sulphate and barium hydroxide is given below:
\(\rm Fe_{2}SO_{4} + 3Ba(OH)_{2} \rightarrow 3BaSO_{4} + 2Fe (OH)_{3}\)
Therefore, the balanced equation of the reaction include the term 2 Fe(OH)₃. Thus, option d is correct.
Find more on balanced reactions:
https://brainly.com/question/14263388
#SPJ2
A marathon is approximately 42.195 km Convert the distance to
centimeters.
Answers
Answer:
4219500 centimeters
Explanation:
I hope this helped!
The water level in a granulated cylinder raised up 6.2 ml after a 16.74 metal sample is lowered into the cylinder. What is the density of the sample? What metal is the sample most likely to be?
Answers
Answer:
The density of the mystery metal is 2.7g/cm^3. It is likely aluminum, Al.
Explanation:
Density is defined as (mass/unit volume). We know the volume of the metal sample by the fact it displaced 6.2 ml of water. Since the mateal sample had a mass of 16.74 grams, we find the density by dividing the mass by the volume:
Density = (mass)/(volume) = (16.74g)/(6.2ml)
Density = 2.7 g/ml
Note that the ubntis for density can vary greatly. kg/cm^3 is a possible unit.
since 1 ml = 1 cm^3, we can also say the density of the metal sample is 2.7 g/cm^3. [This is a more common unit for this type of measurement]
A reference book of densities can be search to find what metals have this density. See the attached excerpt form one such table.
While not definitive, it can be seen that aluminum, Al, is a good candidate for the ID of this metal. It has a density of 2.7 g/cm^3, although different forms may deviate slightly. The metal is most likely aluninum.

it is on SWRO plant with a capacity of 50000m3/day the tds of the feed is 41690ppm implying a chloride ion level of around 23000ppm the temperature of the feed is around 18°C in March and 27°C in September the reject has a tds of 64500ppm . the pressure is 70 bar, that plant started to produce water in June 2003 and corrosion problem appeared already few months of service, two type of corrosion could be established, one being crevice corrosion in 11/2" high pressure connector underneath victauling coupling example the same type of problem that have been corrosion in 316L and 317L high pressure piping seven out of 700 such connector were reported to have suffered this type crevice corrosion after 4 months only, provide the remedy to end the problem
Answers
To address the crevice corrosion issue in the high-pressure connectors and piping of the SWRO plant, several remedies can be considered, A SWRO (Sea Water Reverse Osmosis) plant is a water desalination facility that uses reverse osmosis technology to treat seawater or brackish water and produce freshwater
Material Selection: Evaluate the material compatibility with the operating conditions, especially the chloride ion concentration and temperature. Consider using corrosion-resistant alloys such as duplex stainless steel (e.g., 2205) or super duplex stainless steel (e.g., 2507) that have better resistance to chloride-induced corrosion compared to 316L or 317L stainless steel.
Surface Treatment: Apply appropriate surface treatments to enhance corrosion resistance. Passivation or pickling can remove surface contaminants and create a protective oxide layer on the metal surface, reducing the susceptibility to corrosion.
Design Modifications: Evaluate the design of the connectors and piping to minimize crevices and stagnant areas where corrosion can occur. Smooth transitions, avoiding sharp angles or crevices, can help promote better fluid flow and prevent the accumulation of corrosive substances.
Cathodic Protection: Implement cathodic protection methods, such as impressed current or sacrificial anodes, to protect the connectors and piping from corrosion. This technique involves introducing a more easily corroded metal (anode) to the system, which sacrifices itself to protect the connected metal (cathode) from corrosion.
Monitoring and Maintenance: Regularly monitor the corrosion levels and condition of the connectors and piping. Implement a maintenance program that includes periodic inspections, cleaning, and repairs, if necessary, to prevent corrosion from progressing.
It is important to consult with corrosion experts and engineers who specialize in SWRO plant operations to assess the specific conditions, perform material testing, and provide tailored solutions to mitigate the crevice corrosion problem effectively.
To know more about SWRO (Sea Water Reverse Osmosis), click here, https://brainly.com/question/31556553
#SPJ11
In the following image, atoms are represented by colored circles. Different colors represent different
types of atoms. If atoms are touching, they are bonded.
Which of the following boxes shows a mixture of elements and compounds?
A. D
B. C
C. B
D. A

Answers
What a weird way to organize the answers...
3 facts about the core
Answers
Answer:
The inner core is a hot, dense ball of (mostly) iron. It has a radius of about 1,220 kilometers (758 miles). Temperature in the inner core is about 5,200° Celsius (9,392° Fahrenheit). The pressure is nearly 3.6 million atmosphere (atm).
Explanation:
What is a compound
A.) smog
B.) sea water
C.) air
D.) magnesium
E.)salt
Edmentum answer
Answers
Answer:
B
Explanation:
because sea water taste like Salt
Question 2 of 30
A television commercial shows happy people while describing some medical
symptoms. These symptoms include feeling tired and sad. The medication
being advertised by the commercial was approved by the FDA to treat a
disease that causes these symptoms. The narrator says that it is available by
prescription only and contains 1% of the active ingredient. What can you infer
about this medication?
OA. The people in the commercial are happy because they were
treated by the medication.
B. The medication would be more effective if it contained 10% of the
active ingredient.
C. Anyone who has the symptoms should request a prescription
from his or her doctor.
D. The medication can treat people who have the disease described.
Answers
The medication can treat people who have the disease described. According to the commercial, the drug has FDA approval to treat a condition whose symptoms are listed.
Additionally, the narrator notes that the medication only comes with a prescription and has 1% of the active substance. We can deduce from this information that the drug can effectively treat persons who have the condition generating these symptoms, but obtaining it requires a prescription. The commercial provides no support for the other possibilities.
Therefore, the correct option is D.
Learn more about Medication, here:
https://brainly.com/question/11098559
#SPJ1
Rieun pours 377 g of water at 54°C into an 816-g aluminum container with an initial temperature of 12°C. The specific heat of aluminum is 900 J/(kg ∙ K) and that of water is 4190 J/(kg ∙ K). Assuming no heat is exchanged with the surroundings, find the final temperature of the system in celsius degrees. Please give your answer with one decimal place.
Answers
Answer:
The final temperature of the system, with one decimal place, is 29.5°C.
Explanation:
The heat gained by the aluminum container will be equal to the heat lost by the water. We can use the equation:
Q_aluminum = Q_water
where Q_aluminum is the heat gained by the aluminum container, and Q_water is the heat lost by the water.
The heat gained by the aluminum container can be calculated using the specific heat of aluminum, the mass of the container, and the change in temperature:
Q_aluminum = (mass_aluminum) x (specific_heat_aluminum) x (change in temperature)
Q_aluminum = (816 g) x (0.9 J/(g∙K)) x (final temperature - 12°C)
The heat lost by the water can be calculated using the specific heat of water, the mass of the water, and the change in temperature:
Q_water = (mass_water) x (specific_heat_water) x (change in temperature)
Q_water = (377 g) x (4,190 J/(g∙K)) x (54°C - final temperature)
Since Q_aluminum = Q_water, we can set these two equations equal to each other and solve for the final temperature:
(mass_aluminum) x (specific_heat_aluminum) x (final temperature - 12°C) = (mass_water) x (specific_heat_water) x (54°C - final temperature)
(816 g) x (0.9 J/(g∙K)) x (final temperature - 12°C) = (377 g) x (4,190 J/(g∙K)) x (54°C - final temperature)
Simplifying and solving for final temperature, we get:
final temperature = 29.5°C
To, know more on HEAT refer
https://brainly.com/question/1429452#
#spj11
Please help with Chemistry! Very urgent! I’ll give you 40 points

Answers
Answer: 3. No displacement, zinc is most reactive.
4. Calcium Chloride, Calcium is most reactive.
5. No displacement, Copper is most reactive
6. No displacement, Calcium is most reactive
7. Hydrogen Oxide, Hydrogen is most reactive
8. Carbon oxide, Carbon is most reactive
9. No displacement, Aluminum is most reactive
10. Potassium Kryptide + Lead, no displacement, Potassium is most reactive.
Kyle has a mass of 54 kg and is jogging at a velocity of 3 m/s. What is Kyle’s kinetic energy? (Formula: )
18 J
81 J
162 J
243 J
Answers
Answer:
The answer is 243 JExplanation:
The kinetic energy of an object given it's mass and velocity can be found by using the formula
\(KE = \frac{1}{2} m {v}^{2} \\ \)
where
m is the mass
v is the velocity
From the question
m = 54 kg
v = 3 m/s
The kinetic energy is
\(KE = \frac{1}{2} \times 54 \times {3}^{2} \\ = 27 \times 9 \\ = 243 \: \: \: \: \: \)
We have the final answer as
243 JHope this helps you
Answer:
243 J
Explanation:
JUST GOT IT RIGHT ON EDG
What is the molarity of .65 L of solution containing 63 grams of NaCl? (dont forget to convert grams to moles)
Answers
Answer:
1.66 M
Explanation:
Molarity = moles/L solution
We have the following data:
mass NaCl = m = 63 g
volume of solution = V = 0.65 L
Thus, we first convert the mass to moles with the molar mass of NaCl (MM):
MM(NaCl) = 23 g/mol Na + 35.4 g/mol Cl = 58.4 g/mol
moles NaCl = m/MM = 63 g/(58.4 g/mol)= 1.08 mol
Finally, we divide the moles into the volume of solution to calculate the molarity:
M = moles NaCl/V = 1.66 mol/L
Consider the following pair of reactions. Predict the type of substitution mechanism, predict which reaction of the pair will occur at the faster rate, and draw the correct organic product
Answers
The reaction with S_N₂mechanism is likely to be faster than the reaction with S_N₂ mechanism. This is because the carbocation intermediate formed in S_N₁ mechanism is more stable.
The pair of reactions given below is:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH₃OH + NaI
The type of substitution mechanism:
The first reaction involves S_N₁ mechanism (unimolecular nucleophilic substitution). The second reaction involves S_N₂ mechanism (bimolecular nucleophilic substitution).
Prediction of the reaction that will occur at a faster rate:
The reaction with S_N₁ mechanism is likely to be faster. The rate of this reaction mainly depends on the stability of the carbocation intermediate formed after the initial step. In this case,CH₃Cl reacts to form a tertiary carbocation which is more stable than the primary carbocation formed in CH₃I.
Drawing the correct organic product:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH_3OH + NaI
CH₃C reacts with NaOHin an S_N₁ mechanism to produceCH₃OH and NaCl.
CH₃ reacts withNaOH in an S_N₂mechanism to produce CH₃OH and NaCI.
To know more about unimolecular nucleophilic substitution visit:
brainly.com/question/32657850
#SPJ11
