Answers
Answer: The answer is D \(C_{2} OH_{6}\)
Explanation: Because I just know by counting the letters to see how many of each one.
Answer:
C2OH6
Explanation:
The figure shows the presence of...
Two Carbon (C)
One Oxygen (O)
and Six Hydrogen (H)
We need to make sure we select the option that best represents all of the elements shown in the figure.
Because option 4: C2OH6 is the only chemical formula to include all the elements displayed, it is the best answer.
Related Questions
For each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants.
reactants oxidized _____
reactants reduced _____
a. 2Fe(s)+3Pb(NO3)2(aq)→3Pb(s)+2Fe(NO3)3(aq)
b. AgNO3(aq)+Cu(s)→2Ag(s)+CuNO)2(a)
c. 3AgNO(aq)+Al()→3Ags)+Al(NO3)3(aq)
Answers
Answer:
a. Oxidized: Fe(s)
Reduced: Pb(NO3)2
b.Oxidized: Cu(s)
Reduced: AgNO3
c. Oxidized: Al(s)
Reduced: AgNO3
Explanation:
In a redox reaction, one reactant is been oxidized whereas the other is reduced.. The reduced reactant is the one that is gaining electrons and the oxidized one is loosing electrons.
In the reactions:
a. 2Fe(s)+3Pb(NO3)2(aq)→3Pb(s)+2Fe(NO3)3(aq)
The Fe is as reactant as Fe(s) (Oxidation state 0) and the product is +3 (Because NO3, nitrate ion, is always -1). That means Fe is oxidized. The Pb as reactant is +2 and as product 0 (Gaining 2 electrons). Pb(NO3)2 is reduced
b. 2AgNO3(aq)+Cu(s)→2Ag(s)+Cu(NO3)2(a)
AgNO3 is +1 and Ag(s) is 0. AgNO3 is reduced. Cu(s) is 0 as reactant and +2 as product. Cu(s) is been oxidized
c. 3AgNO3(aq)+Al(s)→3Ag(s)+Al(NO3)3(aq)
Here, in the same way, AgNO3 is +1 as reactant and 0 as product. AgNO3 is reduced. And Al(s) is 0 as reactant but + 3 as product. Al(s) is oxidized.
4. How many grams is 3 moles of H₂O?
Answers
Answer:
1.67
Explanation:
Mass÷mr=moles
3÷18=1.67
Which of the following would be considered a strong base?
a H₂SO4
b. CO₂
c. Ca(OH)₂
d. C₁₂H₂O..
Answers
It’s name is calcium hydroxide and often it is easy to tell if something is a strong base if it has (OH) in the name.
19. Find out the fundamental units involved in the units of
a. velocity
b. acceleration
c. work
d. pressure
e. power
f . density
g. volume
h. force
Answers
Answer: The Fundamental units are as follows:
Velocity: m/secAcceleration: m/sec²Work: kgm²/sec²Pressure: kgm/sec²Power: kgm²/sec³Density: kg/m³Volume: m³Force: kgm/sec²Explanation:
A fundamental unit is a tool used for measurement of a base quantity.
Velocity: It is defined as rate of displacement. Therefore units of displacement and time are involved the units of displacement are same as that of distance i.e. metre and that of time are second. therefore the units of velocity are metre per second.
Acceleration: It is defined as rate of change of velocity. Therefore units of acceleration involve velocity and time. The units of velocity Re metre per second and time is second. Therefore units of acceleration are meter's per second².
Work: It is defined as product of force and displacement. Therefore units of work involve Force and displacement i.e. distance. Therefore units of work are kgm²/sec².
Pressure: It is Force per unit area. Therefore units of Pressure are kg/ms².
Power: It is Work/Time. Therefore units of power are kgm²/sec³.
Density: It is Mass/Volume. Therefore units of density are kg/m³.
Volume: The units of volume are m³.
Force: It is product of mass and acceleration. Therefore units of force are m/sec².
For Further information on Fundamental units
https://brainly.com/question/33348059
Answer: a. meters per second(m/s) b. meters per second squared(m/s2)
c. Joule(J) d.Pascal(Pa) e. Watt(W) f. kilograms per meter cubed(kg/m3)
g. meter cube(m3) h.Newton(N)
Explanation: To find out the fundamental units of the quantities we need to use the SI units of the Fundamental Physical Quantities they are as follows:
Mass:- kg
Length:-m
time:-s
Now we know Velocity = displacement/time which means its units will be m/s,
Acceleration = velocity/time hence its units are m/s2,
Work = force/displacement here units of force is N, therefore, units of work are N/m which is known as Joule(J),
Pressure = force/area where units of area in m2 thus units of pressure are N/m2 which is known as Pascals(Pa),
Power = work/time, therefore, its units are J/s which is known as Watts(W),
density =mass/volume here units of volume are m3 therefore units of density are kg/m3
Volume is a derived unit from length and its units are m3, Force=mass*acceleration thus its units are kg*m/s2 which is known as Newton(N)
Identify two characteristics of water that are a result of its molecular structure. Describe how each characteristic is related to the molecular structure of water.
Answers
Answer:
Explained below
Explanation:
1) Hydrogen bonds are formed between molecules
All these is because the bonds are flexible in such a manner that in the liquid phase, the molecules are relatively close to each other.
However, it is also noted that the hexagonal arrangement of ice is formed the bonds will be locked in place which will result in the average distance between molecules actually increasing which in turn means given a result that ice is less dense than liquid water.
B) Water possesses a high boiling point: This is because the Hydrogen oxygen bond or O-H bonds are highly polar and the Hydrogen atoms in this bond are located at two corners of the tetrahedron shaped like bond shape which gives water it's high molecular polarity in which in turn makes the molecules to stick together.
Which of the following is a homogeneous mixture?l
Answers
Sugar water is a homogeneous mixture. Therefore, option A is correct.
What are the mixtures?A mixture can be described as made up of two or more different substances which are physically combined in a mixture. A mixture of two or more substances can break down into their original components.
The composition of a heterogeneous mixture can not be uniform entire the mixture while the composition of a homogeneous mixture can be always the same.
Pure substances cannot be broken down into simple substances that have only one kind of atom in the entire composition.
A pure substance can be described as made up of two or more elements that are chemically combined together and has a set composition such type of pure substance is called a compound.
As the sugar completely dissolved in water so it is a homogeneous mixture.
Learn more about mixture, here:
brainly.com/question/6243623
#SPJ1
Your question is incomplete, the complete question was,
Which of the following is a homogeneous mixture
A ) sugar solution
B) Mud
C) dirt
D) salsa
А group of students are investigating what happens when you put different
temperatures of water together. In this investigation, they have set up a vial
of colorless room-temperature water and are adding purple hot water at the
bottom of the vial, using a pipette
What do you predict happens in the first 5 seconds.

Answers
Answer:
B. The hot water mixes all through the vial
Explanation:
The hot water is able to mix all through the vial because when water is heated, it's molecule are loosed. They are able to speed up and spread, occupying a larger volume. Hot water is less dense than room-temperature water and the hot water can float on room-temperature water.
If you were to combine two carbon-12 atoms in a fusion process, what element would you get?.
Answers
The element would you get is Mg. 6C12 + 6C12 1 11Na24 + +1e0.
The first step in making carbon is to fuse the nuclei of hydrogen the lightest element to make helium the second lightest element. The next step is to fuse the two helium 4 nuclei. Each contains two protons and two neutrons. Forms beryllium-8. This takes in another helium and produces carbon-12.
The main problem in the carbon cycle is proton addition but after a carbon-12 nucleus fuses with a proton to form nitrogen-13 one of the protons decays to carbon-13 emitting a positron and a neutrino. Two more proton captures produce nitrogen-14 and then oxygen-15. Stellar Fusion Reactors In massive stars hydrogen burns to form helium. This produces carbon which can be further processed into oxygen and heavier elements.
Learn more about The element here:- https://brainly.com/question/18096867
#SPJ4
4. Ms. Desabille bought pork meat for P205.00 per kilo , about how much did she pay for 7.5 kilos?
A. P 1500.00 B. P1600,00
C. P 1400.00
DP 1300.00
5. If gasoline costs P49.65 per liter and your tank holds 50.35 liters , then about how much will you pay to full
your tank?
A P 2 500.00 B. P2 400.00
C. P2 300.00
D. P 1 500.00
6. Mr. Bernard travels 5.12 km per hour on his bike. How far can he travel in 3.25 hours?
A 1.664 km B. 166.4 km
C. 16.64 km
D. 1664
7. Mrs. Allysa purchased 48 pencils at P 4.50 each. How much did she pay for all the pencils?
A P 261.00 B. P 162.00
B. P 216.00
D. 612.00
8. The Villaroel family bought a 125.65 square meter lot at P 4500.00 per square meter. How much did they
pay?
A. P556 425 B. P 565 425
C. P 556 452
D. P 655 425
9. What is 2.7 - 10?
A 2.70
B. 0.027
C. 0.27
D. 27
10. What is 98.75 + 4?
A. 24. 6877 B. 24.6885
C. 24.6865
D. 24.6875
11. A store owner has 63 kilograms of candy. If she puts the candy into 21 jars, how much candy will each ja
contain?
A. 4 kg
B. 3 kg
C. 0.4 kg
D. 0.3 kg
12. It is the quantitative relation between two amounts showing the number of times one value contains or is
contained within the other.
A. proportion B. ratio
C. number
D. quantity
math ito
brainliest
Answers
Nurse Antonio measured out 7 grams of sodium chloride (NaCI). Using dimensional analysis, calculate how many moles of NaCI he weighed out.
Answers
Answer:
0.119 moles
Explanation:
Given that,
Mass measured by Nurse Antonio is 7 grams of NaCl
To find,
The no of moles of NaCl
Solution,
The number of moles is given by
\(n=\dfrac{m}{M}\)
m is given mass
M is molar mass
For NaCl, molar mass is 23+35.5 = 58.5 grams
So,
\(n=\dfrac{7}{58.5}\\\\n=0.119\ \text{moles}\)
Therefore, there are 0.119 moles of NaCl.
what is the concentration of a nitric acid solution if 10.0 ml of the solution is neutralized by 3.6 ml of 0.2 m naoh?
Answers
Answer:
The concentration of the nitric acid (HNO3) solution is 72 M.
Explanation:
To determine the concentration of the nitric acid solution, we can use the concept of stoichiometry and the equation of the neutralization reaction between nitric acid (HNO3) and sodium hydroxide (NaOH):
HNO3 + NaOH → NaNO3 + H2O
The balanced equation shows that the molar ratio between HNO3 and NaOH is 1:1. This means that 1 mole of HNO3 reacts with 1 mole of NaOH.
Given:
Volume of HNO3 solution = 10.0 ml
Volume of NaOH solution = 3.6 ml
Molarity of NaOH solution = 0.2 M
To find the concentration of the HNO3 solution, we need to calculate the number of moles of NaOH used in the neutralization reaction:
moles of NaOH = volume of NaOH solution * molarity of NaOH solution
= 3.6 ml * 0.2 M
= 0.72 mmol (millimoles)
Since the molar ratio between HNO3 and NaOH is 1:1, the number of moles of HNO3 in the solution is also 0.72 mmol.
Now, we can calculate the concentration of the HNO3 solution using the formula:
concentration (in M) = moles of solute / volume of solution (in L)
concentration = 0.72 mmol / 0.010 L
= 72 mmol/L
= 72 M
Therefore, the concentration of the nitric acid (HNO3) solution is 72 M.
How do you balance this equation?

Answers
Answer:
HC₂H₃O₂ + NaHCO₃ —> NaC₂H₃O₂ + CO₂ + H₂O
The coefficients are: 1, 1, 1, 1, 1
Explanation:
_HC₂H₃O₂ + _NaHCO₃ —> _NaC₂H₃O₂ + _CO₂ + _H₂O
To balance an equation, we simply do a head count of the individual elements and ensure they are balanced on both side.
For the above equation, we shall balance it as :
HC₂H₃O₂ + NaHCO₃ —> NaC₂H₃O₂ + CO₂ + H₂O
Reactant:
H = 5
C = 3
O = 5
Na = 1
Product:
H = 5
C = 3
O = 5
Na = 1
From the above, we can see that each element is the same on both side of the equation. Thus the equation is already balanced
HC₂H₃O₂ + NaHCO₃ —> NaC₂H₃O₂ + CO₂ + H₂O
The coefficients are: 1, 1, 1, 1, 1
1. What organ system is responsible for controlling all of the body
functions?
Answers
Answer:
The human brain is the body's control center, receiving and sending signals to other organs through the nervous system and through secreted hormones. It is responsible for our thoughts, feelings, memory storage and general perception of the world. The human heart is a responsible for pumping blood throughout our body
An object has a mass of 40.1g and occupies a volume of 7.19mL. The density of this object is
A. Too low to measure
B. 288 g/mL
C. 5.58 g/mL
D. 40.1 g/mL
E. 0.179 g/mL
Answers
Answer:
5.58 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{40.1}{7.19} \\ = 5.57719 ...\)
We have the final answer as
5.58 g/mLHope this helps you
Question 2 of 10
What is the percent yield of a reaction?
The amount of product obtained x 100
amount possible
B. The amount of product actually obtained in a reaction
C. The amount of product that is possible from a reaction
D. The difference between measured and calculated amounts
A.
Answers
Answer:
c
Explanation:
A molecule is made up of 1 atom of potassium, 1 atom of chlorine, and 3 atoms
of oxygen. What is the molecular formula of this molecule?
Answers
Answer:
I think you should go read up on the periodic table
Consider the following reversible reaction
What is the equilibrium constant expression for the given system?

Answers
Answer:
Explanation:
a
Answer:
The answer is D - Keq = [H2]^2 × [O2]
[2]^2
Explanation:
The equilibrium constant (Keq), is a quantitative measure of the position of equilibrium in a chemical reaction. It is defined by the ratio of the concentrations (or partial pressures) of the products to the concentrations (or partial pressures) of the reactants.
For a general chemical reaction:
aA+bB + CC+dD
Explanation: The equilibrium constant expression is given by:
Keq = [C]^c x [D]^d
[A]^a x [B]^b
Where [A], [B], [C], and [D] represent the molar concentrations of the species A, B, C, and D, respectively. The coefficients a, b, c, and d represent the stoichiometric coefficients of the balanced equation.
The given reaction is 2H2O(g) ↔ 2H2(g) + O2(g)
The equilibrium constant expression for the given system can be expressed as follows:
Keq = [H2]^2 × [O2]
[2]^2
Where:
[H2] represents the molar concentration of hydrogen gas (H2).
[O2] represents the molar concentration of oxygen gas (O2).
[H2O] represents the molar concentration of water vapor (H2O).
The equilibrium constant expression for the given system can be expressed as follows: Keq = [H2]^2 × [O2]
[2]^2
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
Text: Changing Land
1. What are three agents of change responsible
for changing landforms?
2. According to the text, what are 3 landforms
created by wind?
3. According to the text, what 2 landforms are
created by glaciers?
4. According to the text, what landform is formed
by deposition at the mouth of a river?
5. U-shaped valleys and canyons are both formed
by weathering and erosion. What is another
landform that is created due to weathering
and erosion?
Plissss help now
Is for cience
Answers
The three main causes of erosion, or the removal of soil, rock, and other materials, are wind, water, and ice.
What three factors lead to changes in landforms?Compared to plate tectonics, landforms are changed much more quickly by earthquakes, weathering, and people, and these changes are frequently visible.
Sand dunes, Loess Deposits, Yardangs, Ventifact, Deflation Hollow or Blowout, and Desert Pavement are among the geological features.
Glaciers carved a collection of strange valleys with flat bottoms and steep walls. Hanging valleys, fjords, and U-shaped valleys are a few examples of the different sorts of valleys that glaciers can destroy.
Alpine glaciers have their origins in the mountains, in a number of our National Parks. When they form in tiny basins with sloping sides, they are referred to as cirque glaciers (cirques).
U-shaped valleys have been found to be produced by glacial erosion. A massive glacier's journey through the landscape leaves imposing traces. Walls of rock blocks are torn apart by its abrasive force.
To learn more about landforms refer to:
https://brainly.com/question/20932760
#SPJ1
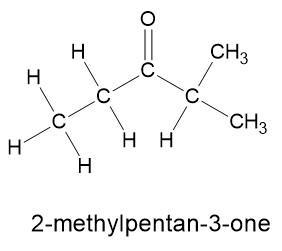
СН3-СН2-С — Н
Spell out full name
Answers
Answer:
2-methylpentan-3-one
Hope this helps.
true or false energy cannot be changed into different forms
Answers
Answer:
False
Explanation:
Energy can be changed into different forms
Estimate the pressure in atm at which the solid, liquid, and gas phases of SO_2 can coexist at the same time?
Answers
The triple point pressure or the pressure in atm at which the solid, liquid, and gas phases of SO₂ can coexist at the same time is 0.0165 am
What is the triple point of a substance?The triple point of a substance is the temperature and pressure at which a substance can exist in equilibrium in the liquid, solid, and gaseous states.
Different substances have different triples points.
The triple point of pure water is at 0.01°C and 4.58 mmHg.
The triple point of sulfur dioxide is 197.64 K and 0.0165 atm.
Therefore, the pressure in atm at which the solid, liquid, and gas phases of SO₂ can coexist at the same time is 0.0165 am
In conclusion, the triple point of substance varies.
Learn more about triple point at: https://brainly.com/question/2402164
#SPJ1
A 5.22 × 10−3−mol sample of HY is dissolved in enough H2O to form 0.088 L of solution. If the pH of the solution is 2.37, what is the Ka of HY?
Answers
Answer:
3.07 × 10⁻⁴
Explanation:
Step 1: Calculate the concentration of H⁺
We will use the definition of pH.
\(pH = -log [H^{+} ]\\\[ [H^{+} ] = antilog -pH = antilog -2.37 = 4.27 \times 10^{-3} M\)
Step 2: Calculate the concentration of HY
5.22 × 10⁻³ mol of HY are dissolved in 0.088 L. The concentration of the acid (Ca) is:
\(Ca = \frac{5.22 \times 10^{-3} mol }{0.088L} = 0.0593M\)
Step 3: Calculate the acid dissociation constant (Ka)
We will use the following expression.
\(Ka = \frac{[H^{+}]^{2} }{Ca} = \frac{(4.27 \times 10^{-3} )^{2} }{0.0593} = 3.07 \times 10^{-4}\)
which property is least helpful in identifying a sample of matter? A. melting point B. Volume. C. Reactivity. D. Boiling point
Answers
Answer:
which property is least helpful in identifying a sample of matter is (B) VOLUME
Explanation:
volume depend on the amount of substance present and are not useful in the identification of a sample of matter. volume is an extensive property so it is not useful identifying the sample of matter.
please help i am losing motivation and very depressed please look at photo and answer.

Answers
Answer:
Solid turns to liquid.
Explanation:
When you freeze water, it turns into ice. When melted it's still water. No chemical changes were made to the piece of ice melted. The only change was ice (solid) to water (liquid)
Conservation of Matter - Matter can change forms through physical and chemical changes, but no matter what, matter is conserved. The same amount of matter exists before and after the change.
Hope this helps! Please mark brainliest!
Define and explain the causes of climate change
Answers
Answer:
The primary cause of climate change is the burning of fossil fuels, such as oil and coal, which emits greenhouse gases into the atmosphere—primarily carbon dioxide. Other human activities, such as agriculture and deforestation, also contribute to the proliferation of greenhouse gases that cause climate change
Is medicine, such as the aspirin shown in the image above, an example of science or technology?
Answers
Answer:
Technology
hope this helps
The chemical reaction between water and magnesium is?
Answers
Answer: hydrogen gas
Explanation: When magnesium interacts with water, it will form a hydrogen gas that ignites violently due to the excessive heat and oxygen supply.
PRACTICE PROBLEM Two compounds, A and B, have the same molecular formula, C6H8. Both A and B react with two molar equivalents of hydrogen in the presence of platinum to yield cyclohexane. Compound A shows three signals in its broadband decoupled NMR spectrum. Compound B shows only two NMR signals. Compound A shows an absorption maximum at 256 nm, whereas B shows no absorption maximum at wavelengths longer than 200 nm. What are the structures of A and B
Answers
Answer:
See explanation
Explanation:
One basic thing that we must keep in mind is that A and B are both dienes.
The NMR spectrum and absorption maxima of A and B indicates that A may be a conjugated diene while B may be an isolated diene.
Remember that conjugated dienes shift the absorption maxima to longer wavelengths due to π - π* transition.
The two structures attached may suffice for compounds A and B

If it takes 54 mL of 0.1 M NaOH to neutralize 125 mL HCl, what is the concentration of the HCl solution
Answers
The concentration of the HCl solution needed to neutralise NaOH is 0.0432M
Neutralization is a chemical reaction in which acid and base react to form salt and water. Hydrogen (H⁺) ions and hydroxide (OH⁻ ions) react with each other to form water.
The strong acid and strong base neutralization have a pH value of 7.
The beaker gets warm which indicates that the reaction between acid and base is an exothermic reaction releasing heat energy into the surroundings.
Given,
Volume of NaOH = 54ml
Concentration of NaOH = 0.1 M
Volume of HCl = 125ml
Concentration of NaOH × Volume of NaOH = Concentration of HCl × volume of HCl
0.1 × 0.054 = Concentration of HCl × 0.125
Concentration of HCl = 0.0432 M
Learn more about Neutralization, here:
https://brainly.com/question/13913970
#SPJ1
