Pls predict and balance the following Chemical equations 30points (5 per question)
Will mark Brainliest
1. Na2(SO4) (aq) + Ba(NO3)2(aq) -->
2. Na(NO3)(aq) + NH4Cl(aq) -->
4. ZnCl2 (aq) + K (SO4) (aq) -->
5. ZnCl2 (aq) + Na2S (aq) -->
6. Na(C2H3O2) (aq) + Ag(NO3) (aq) -->
Answers
Answer:
1. Na2SO4 + Ba(NO3)2 → NaNO3 + BaSO4
2. NaNO3 + NH4Cl → NaCl + NH4NO3
4. Sorry, I don't know
5. Refer to the attachment..
6. NaC2H3O2(aq) + AgNO3(aq) = NaNO3(aq) + AgC2H3O2
Hope it can help you and please mark me as a brainlist...\( \huge{\blue{Thank \: you}}\)

1. Na2(SO4) (aq) + Ba(NO3)2(aq) -->
Answer: NaNO3 + BaSO4 - Chemical Equation Balancer.
2. Na(NO3)(aq) + NH4Cl(aq) -->
Answer: NaNO3 + NH4Cl → NaCl + NH4NO3 - Balanced equation
4. ZnCl2 (aq) + K (SO4) (aq) -->
Answer: (This is an impossible reaction)
5. ZnCl2 (aq) + Na2S (aq) -->
Answer: no reaction occurs
6. Na(C2H3O2) (aq) + Ag(NO3) (aq) -->
Answer: NaC2H3O2(aq) + AgNO3(aq) = NaNO3(aq) + AgC2H3O2
(I’m not sure if all are right, I’m sorry but this is the best I could do. Hope it helps you.)
Related Questions
PLEASE HELP ME ASAP
An ion has 16 protons, 17
neutrons, and 18 electrons. What
is the correct isotope notation?
A. As-2 B. CI-1
33
17
C. 335-2 D. 32S-2
16
16
Enter the answer choice letter.

Answers
Answer: d
Explanation:
Please people help WHAT IS THE ANSWER? NO LINKS PLEASE

Answers
stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
If you react 10.0 mL of 1.5 M CoCl₂ with plenty of NaOH, how many grams of Co(OH)₂ will precipitate out?
Answers
1.4 g Co(OH)₂
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Unit 0
Base 10 decimal systemAtomic Structure
Reading a Periodic TableMolarity = moles of solute / liters of solutionAqueous Solutions
States of MatterPrediction Reactions RxNStoichiometry
Using Dimensional AnalysisAnalyzing Reactions RxNExplanation:Step 1: Define
[RxN - Balanced] CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
[Given] 10.0 mL, 1.5 M CoCl₂
[Solve] grams Co(OH)₂
Step 2: Identify Conversions
[Base 10] 1000 mL = 1 L
[RxN] 1 mol CoCl₂ → 1 mol Co(OH)₂
[PT] Molar Mass of Co - 58.93 g/mol
[PT] Molar Mass of O - 16.00 g/mol
[PT] Molar Mass of H - 1.01 g/mol
Molar Mass of Co(OH)₂ - 58.93 + 2(16.00) + 2(1.01) = 92.95 g/mol
Step 3: Stoich
[DA] Convert mL to L [Set up]: \(\displaystyle 10.0 mL(\frac{1 \ L}{1000 \ mL})\)[DA] Multiply/Divide [Cancel out units]: \(\displaystyle 0.01 \ L\)[DA] Find moles of CoCl₂ [Molarity]: \(\displaystyle 1.5 \ M \ CoCl_2 = \frac{x \ mol \ CoCl_2}{0.01 \ L}\)[DA] Solve for x [Multiplication Property of Equality]: \(\displaystyle 0.015 \ mol \ CoCl_2\)[DA] Set up [Reaction Stoich]: \(\displaystyle 0.015 \ mol \ CoCl_2(\frac{1 \ mol \ Co(OH)_2}{1 \ mol \ CoCl_2})(\frac{92.95 \ g \ Co(OH)_2}{1 \ mol \ Co(OH)_2})\)[DA] Multiply/Divide [Cancel out units]: \(\displaystyle 1.39425 \ g \ Co(OH)_2\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs as our lowest.
1.39425 g Co(OH)₂ ≈ 1.4 g Co(OH)₂
Answer:
1.4 g Co(OH)₂
Explanation:
Molar mass of Co(OH)₂ = (58.9 + 16.0×2 + 1.0×2) g/mol = 92.9 g/mol
According to the given equation, mole ratio CoCl₂ : Co(OH)₂ = 1 : 1
No. of moles of CoCl₂ reacted = (1.5 mol/L) × (10.0/1000 mol) = 0.015 mol
No. of moles of Co(OH)₂ precipitated = 0.015 mol
Mass of Co(OH)₂ precipitated = (0.015 mol) × (92.9 g/mol) = 1.39 g / 1.4g
====
OR:
(1.5 mol CoCl₂ / 1000 mL CoCl₂ solution) × (10.0 mL CoCl₂ solution) × (1 mol Co(OH)₂ / 1 mol CoCl₂) × (92.9 g Co(OH)₂ / 1 mol Co(OH)₂)
= 1.39 g Co(OH)₂ / 1.4 g Co(OH)₂
A student adds 4.00 g of dry ice (solid ) to an empty balloon. What will be the volume of the balloon at STP after all the dry ice sublimes (converts to gaseous )
Answers
The volume of the balloon at STP after all the dry ice sublimes will be determined by the amount of gas produced.
What determines the balloon's volume?In the process of sublimation, solid dry ice directly converts into carbon dioxide gas without passing through a liquid phase. The molar mass of carbon dioxide is approximately 44 g/mol. To calculate the number of moles of carbon dioxide produced, we divide the mass of dry ice (4.00 g) by its molar mass.
4.00 g / 44 g/mol = 0.091 mol
At STP (Standard Temperature and Pressure), which is 0 degrees Celsius (273.15 K) and 1 atmosphere of pressure, one mole of any ideal gas occupies 22.4 liters. Therefore, the number of moles of carbon dioxide (0.091 mol) will occupy:
0.091 mol x 22.4 L/mol = 2.0464 L
Hence, the volume of the balloon at STP after all the dry ice sublimes will be approximately 2.05 liters.
Sublimation is the process in which a substance transitions directly from a solid to a gas phase without becoming a liquid. In the case of dry ice, which is solid carbon dioxide, the sublimation occurs at temperatures below -78.5 degrees Celsius (-109.3 degrees Fahrenheit) and atmospheric pressure. The conversion from solid to gas leads to an expansion in volume, making it suitable for inflating balloons. The molar mass of carbon dioxide is used to determine the number of moles of gas produced, which is then multiplied by the molar volume at STP (22.4 L/mol) to find the resulting volume. This calculation assumes ideal gas behavior and standard conditions.
Learn more about dry ice
brainly.com/question/30492237
#SPJ11
A 47. 95 mL sample of a 0. 200 M solution of barium nitrate is mixed with 18. 25 mL of a 0. 250 M solution of potassium sulfate. Assuming that all ionic species are completely dissociated and the temperature is 25 degrees C, what is the osmotic pressure of the mixture in torr
Answers
Osmotic pressure of the mixture, we can use the formula Π = MRT Π is the osmotic pressure, M is the molarity of the solution, R is the ideal gas constant (0.0821 L·atm/(mol·K)), T is the temperature in Kelvin.
First, we need to calculate the total moles of solute in the mixture.
For the barium nitrate solution:
Volume (V1) = 47.95 mL = 0.04795 L
Molarity (M1) = 0.200 M
Moles of solute in barium nitrate solution = M1 × V1 = 0.200 mol/L × 0.04795 L = 0.00959 mol
For the potassium sulfate solution:
Volume (V2) = 18.25 mL = 0.01825 L
Molarity (M2) = 0.250 M
Moles of solute in potassium sulfate solution = M2 × V2 = 0.250 mol/L × 0.01825 L = 0.00456 mol
The total moles of solute in the mixture = Moles of solute in barium nitrate solution + Moles of solute in potassium sulfate solution = 0.00959 mol + 0.00456 mol = 0.01415 mol
Now, we can convert the temperature to Kelvin:
Temperature (T) = 25°C + 273.15 = 298.15 K
Using the formula Π = MRT, we can calculate the osmotic pressure (Π):
Π = (0.01415 mol/L) × (0.0821 L·atm/(mol·K)) × (298.15 K)
Π = 0.346 atm
To convert the osmotic pressure to torr, we can use the conversion factor:
1 atm = 760 torr. Therefore, the osmotic pressure of the mixture is approximately:
0.346 atm × 760 torr/atm = 263.36 torr.
Learn more about Osmotic pressure here
https://brainly.com/question/29823250
#SPJ11
in the lab we heated auger. Which type of bond did the sugar have
Answers
Sugar is a simple carbohydrate consisting of carbon, oxygen, and hydrogen molecules which are linked by covalent bonds.
What are the chemical covalent bonds?The chemical covalent bonds are a type of chemical bond in which atoms that differ in their electronegativity share electrons to maintain cohesion i.e., intramolecular cohesion, while sugar molecules may interact by glycosidic bonds.
Therefore, with this data, we can see covalent bonds bind atoms in the sugar molecule while glycosidic bonds are those required to connect different sugar and thus form a complex macromolecule or polysaccharide.
Learn more about chemical covalent bonds here:
https://brainly.com/question/3447218
#SPJ1
Which of the following will conduct electricity?
Question 9 options:
A) Acid solutions only
B) Base solutions only
C) Acid solutions and base solutions
D) Neither acid solutions nor base solutions
Answers
Answer:
I think is c
Explanation:
I hope this helps you
Answer:
Acid solutions and base solutions
Explanation:
Use your answers in Part A and Part B to compare the differences in temperatures between the two sites.

Answers
Answer:
uhhh
Explanation:
uhhh
Min increases the temperature of a gas in an expandable container. If she keeps the pressure constant, what will happen to the volume of the gas?
Answers
Answer:
The volume will remain the same.
Explanation:
The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law)
There are so many gas laws like Charles's law, Avogadro’s law, Boyle 's law and many more. If pressure of gas is kept constant, then volume of the gas will be increasing.
What is Charles's law?According to Charles's law, gas expands on heating. At constant pressure, volume well be directly proportional to temperature.
According to ideal gas equation
PV=nRT
On keeping pressure constant the following equation can be deduced to Charles's law as
V₁÷T₁=V₂÷T₂
where,
V₁= initial volume
V₂= final volume
T₁=initial temperature
T₂=final temperature
According to Charles's law, the volume of a given gas sample is directly proportional to its absolute temperature at constant pressure.
Therefore, if pressure is kept constant, then volume of the gas will be increasing.
To know more about Charles's law, here:
https://brainly.com/question/16927784
#SPJ2
.
43. Standard enthalpy is measured at
a. 1 atm and 100 degrees C
b. standard atmospheric pressure and standard state
c. room temperature and one atm
d. both b and c
Answers
Answer:
d. both b and c
Explanation:
Standard enthalpy is typically measured at standard atmospheric pressure and standard state conditions, which means a pressure of 1 atmosphere and at a specified temperature that may vary depending on the context. However, it is common to use room temperature (around 25 degrees Celsius or 298 Kelvin) as the standard temperature for measuring enthalpy. Therefore, the standard enthalpy is measured at both standard atmospheric pressure and standard state conditions, as well as at room temperature and 1 atmosphere.
A saline solution contains 0.90g of sodium chloride dissolved in 250 cm3 water
(a)what is the amount in moles of sodium chloride dissolved?
(b) the concentration of the saline solution in mol/dm3?
Answers
Explanation:
A. mole=mass/molar mass
Molar mass of NaCl=23+35.5g
NaCl=58.5g
from
mole=0.90/58.5mol
mole=0.90g/58.5mol
mole=0.15
B. concentration=number of moles of solute/volume of solution
concentration=0.15mol/250cm^3
1dm^3=1000cm^3
250=0.25
concentration=0.6mol/dm^3
The __________________ in na ions to the ______________ of the cell leads to polarization and the __________________ of k ions to the ______________ of the cell leads to repolarization.
Answers
The influx of Na+ ions to the interior of the cell leads to polarization and the efflux of K+ ions to the exterior of the cell leads to repolarization.
During an action potential in a neuron, there is a sequential change in the movement of ions across the cell membrane. The depolarization phase is initiated by the opening of voltage-gated Na+ channels, allowing Na+ ions to enter the cell. This influx of positive ions depolarizes the cell membrane, making the interior more positive.
As the depolarization phase continues, voltage-gated K+ channels open, allowing K+ ions to leave the cell. The efflux of K+ ions leads to repolarization, restoring the negative charge inside the cell.
So, the influx of Na+ ions to the interior of the cell leads to polarization, and the efflux of K+ ions to the exterior of the cell leads to repolarization.
Learn more about Na+ ions here:
https://brainly.com/question/4179719
#SPJ11
During chemiosmosis, hydrogen ions accumulate in the ______ of the mitochondrion to create a large electrochemical gradient for aerobic cellular respiration.
Answers
In the course of chemiosmosis, hydrogen ions build up in the mitochondrion's intramembrane space to produce a significant electrochemical gradient for aerobic cellular respiration.
What is the chemiosmosis procedure?Protons are pumped through specialized channels in the mitochondrial membranes from the inner to the outer compartment during chemiosmosis. A gradient of proton (H+) is created by the pumping. Protons diffuse through a transport protein called ATP synthase to diffuse down the gradient once it has been formed.
How is ATP produced through chemiosmosis?Electrons are transferred from one molecule to another in the electron transport chain, and the energy released during these electron transfers is used to create an electrochemical gradient. The energy held in the gradient is utilized in chemiosmosis to produce ATP.
To know more about chemiosmosis visit:
https://brainly.com/question/15443163
#SPJ4
Concentration of a Drug in the Bloodstream The concentration of a certain drug in a patient's bloodstream thr after injection is given by 0.2t C (t) = +2 +1 mg/cm² Evaluate lim C (t) and interpret your < > result.
Answers
the drug concentration will not stabilize in the patient's bloodstream and will continue to increase indefinitely, which could have adverse effects on the patient.
The given drug concentration formula is C(t) = 0.2t + 2 + 1 mg/cm². To find lim C(t), we need to evaluate the limit as t approaches infinity. As t increases without bound, the 0.2t term dominates the equation, making the other two terms negligible. Therefore, lim C(t) = infinity. This means that the drug concentration in the patient's bloodstream will continue to increase indefinitely, which can be a cause for concern if the drug is not properly metabolized or excreted from the body. It is important for healthcare professionals to monitor drug concentrations in patients to avoid toxicity or adverse effects. To find the limit as t approaches infinity, lim C(t), we can analyze the function. As t increases, the 0.2t term will dominate the constant term, 2. Therefore, the concentration of the drug in the bloodstream will keep increasing without bounds as time goes on. Mathematically, lim (t→∞) C(t) = ∞. This result indicates that the drug concentration will not stabilize in the patient's bloodstream and will continue to increase indefinitely, which could have adverse effects on the patient.
To know more about drug visit:
https://brainly.com/question/29767316
#SPJ11
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. What % of the chair conformers have an axial methyl group?
A) 95 B) 75 C) 50 D) 25 E) 5
Answers
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. The % of the chair conformers having an axial methyl group is A) 95%.
If the value of Keq is higher than 1, that means the reaction is favored in the forward direction. If the value of Keq is lower than 1, that means the reaction is favored in the reverse direction.If the value of Keq is equal to 1, that means the reaction is at equilibrium.Methylcyclohexane is an example of the cyclohexane molecule in which there is a substitution of a methyl group on one of the six carbon atoms in the ring structure. The two chair conformers of methylcyclohexane are the axial and equatorial conformers, in which the methyl group is either in an axial or equatorial position, respectively.
The percentage of chair conformers having an axial methyl group can be calculated using the following formula:
% axial conformer = Keq / (1+Keq) × 100
Putting the value of Keq in the formula:
axial conformer = 18 / (1+18) × 100%
axial conformer = 18/19 × 100%
axial conformer = 94.7%
The value of percentage has been rounded off to the nearest whole number, which is 95. Therefore, the answer is (A) 95.
To learn more about equatorial position check the link below-
https://brainly.com/question/32759602
#SPJ11
Temperature is the measurement of how hot or cold something is. Agree Disagree
Answers
Answer:
Agree
Explanation:
Answer:
Agree
Explanation:
Rank the following from MOST soluble in water to LEAST soluble in water.
a) CH3CH2CH2OH
b) HOCH2CH2OH
c) CH3CH2CH2CH3
Answers
Propanol, ethylene glycol, then butane this is the solubility in water of these molecules. Ethylene glycol is miscible in water. Solubility of butane is 61 mg/l. And propanol is totally soluble in water.
What is solubility ?The capacity of a material, the solute, to combine with another substance, the solvent, is known as solubility. Insolubility, or the solute's inability to create such a solution, is the opposite attribute.
Hydrogen bonds as well as dipole-dipole forces can be used by 1-propanol to interact with water. By neither method can butane interact with water. Therefore, 1-propanol has a substantially higher solubility.
The most soluble form of ethylene glycol is water. because it may create a H bond with water because to its two -OH groups. Therefore, more H bonding increases solubility in water.
Thus, most soluble in water to least soluble in water is option A, option B and then option C.
To learn more about solubility follow the link;
https://brainly.com/question/8591226
#SPJ1
_AICI3–> _AI + _Cl2
I need help
Answers
Answer:
2AlCl₃ ⇒ 2Al + 3Cl₂
Explanation:
Balance the equation–making sure there's an equal amount of reactants and products on both sides.
ASAP PLEASE ITS FOR MY FINAL!!!!
How would you go about calculating the molarity of the following solution: 19.5 grams of Be(OH)2 is dissolved in enough water to make a 1.5 L solution.
Answers
Answer:
0.302M
Explanation:
Molarity, which is the molar concentration of a solution can be calculated as follows:
Molarity = number of moles (n) ÷ volume (V)
According to this question, 19.5 grams of Be(OH)2 is dissolved in enough water to make a 1.5 L solution.
Using mole = mass/molar mass to calculate the number of moles in 19.5g of Be(OH)2
Molar mass of Be(OH)2 = 9 + (16 + 1)2
= 9 + (17)2
= 9 + 34
= 43g/mol
mole = 19.5/43
mole = 0.453moles
Molarity = n/V
Molarity = 0.453/1.5
Molarity = 0.302M
convert 7.9 x 10^22 atoms of Ca to grams
Answers
Answer:
Mass = 5.24 g
Explanation:
Given data:
Number of atoms of Ca = 7.9×10²² atoms
Mass of Ca = ?
Solution:
First of all we will calculate the number of moles by using Avogadro number.
1 mole contain 6.022×10²³ atoms
7.9×10²² atoms × 1 mol /6.022×10²³ atoms
1.31 ×10⁻¹ mol
0.131 mol
Mass of Ca:
Mass = number of moles × molar mass
Mass = 0.131 mol × 40 g/mol
Mass = 5.24 g
Calculate the volume percentage of phase present in an alloy of
16% by weight silicon and 84% by weight aluminium. Given density of
Si = 2.35 gm/cc and density of aluminium = 2.7 gm/cc
Answers
The volume percentage of silicon in the alloy is approximately 38.2%.
To calculate the volume percentage of silicon in the alloy, we need to consider the weight percentage and the densities of silicon and aluminium.
First, we calculate the volume of each component in the alloy based on their weight percentages. Since the density is defined as mass per unit volume, we can use the weight percentage to determine the mass of each component. For example, in 100 grams of the alloy, we have 16 grams of silicon and 84 grams of aluminium.
Next, we calculate the volume of silicon and aluminium by dividing their respective masses by their densities. Using the density of silicon (2.35 gm/cc), we find that the volume of silicon is approximately 6.81 cc. Similarly, using the density of aluminium (2.7 gm/cc), we find that the volume of aluminium is approximately 31.11 cc.
Finally, we calculate the volume percentage of silicon in the alloy by dividing the volume of silicon by the total volume of the alloy (sum of the volumes of silicon and aluminium) and multiplying by 100. In this case, the volume percentage of silicon in the alloy is approximately 38.2%.
Learn more about silicon
brainly.com/question/28213172
#SPJ11
The theoretical yield and the percent yield are calculated shown below. Did you perform the calculations correctly?

Answers
Answer:
\(56 \times { \frac{01514344}{?} }^{2} 5566648443hffii51 \\ \div 232333\)
Answer:
write a letter to the presiding member of your district assessment telling him or her about two of the achievement of your community over the last five years and the plans for the future
The galaxy we live in is the Milky Way galaxy, and it is a spiral galaxy. Galaxies contains billions of which of the following?
A.Smaller galaxies
B.Black holes
C.Planets
D.Stars
Need help ASAP
Answers
Answer:
D. Stars is the correct answer
Please give my answer the brainliest...
Describe the journey that a carbon atom from inside a volcano will take to become a carbon atom inside carbonate rock.
Answers
The journey of a carbon atom from inside a volcano to inside a carbonate rock is as follows:
Throughout this journey, the carbon atom undergoes various chemical reactions and changes in form, but ultimately ends up as a component of carbonate rock.
Learn more about carbonate rock at: https://brainly.com/question/12971138
#SPJ11
what is the molar solubility of sat. calcium hydroxide solution with 20.00g ca(no3)2 in 100.00ml?
Answers
The molar solubility of saturated calcium hydroxide solution with stated amount of calcium nitrate is option (a) \( {1.063 × 10}^{-3} \).
The expression to be used for calculation is -
Ksp = [\( {Ca}^{2+} \)\( {OH}^{2-} \)]
As the Ksp is already given, we need to calculate the calcium ion concentration to proceed further
Calcium ion concentration from Ca \( NO_{3}\)\( _{2}\) × 1 mol/164.09 grams
Calcium ions = 0.1219 mol
Concentration in stated volume = 0.1219/100
Concentration = 1.219 M
Finding the hydroxyl ion concentration now -
\( {5.5×10}^{-6} \) = 1.219 × \( {OH}^{2-} \)
Dividing both sides by 1.219, we get -
\( {OH}^{2-} \) = \( {2.126 × 10}^{-3} \)
The solution contains 2 moles of hydroxyl ions. Hence, the molar solubility will be- \( {2.126 × 10}^{-3} \)/2
Molar solubility = \( {1.063 × 10}^{-3} \)
The correct answer is option (a).
Learn more about Ksp-
https://brainly.com/question/29610286
#SPJ4
The complete question is -
What is the molar solubility of sat. calcium hydroxide solution with 20.00g Ca(NO3)2 in 100.00ml? Ksp for Ca(OH)2 = 5.5 x 10^-6 a. 1.062 x 10^-3M b. 0.00276M c. 0.180M d. 5.61M e. 5.61x10^-5M f. 1.172 x 10^-4M
What do these two changes have in common?
burning food on a stove
dry ice sublimating and becoming a gas
Answers
Both burning food on a stove and dry ice sublimating and becoming a gas are examples of physical and chemical changes.
Physical and chemical changes are two types of changes that occur in matter. A physical change is a change in which the form or appearance of a substance changes, but it does not become a different substance.
On the other hand, chemical change is a change in which one substance is transformed into a new substance with different physical and chemical properties.In the given two changes, burning food on a stove and dry ice sublimating and becoming a gas are the examples of physical and chemical changes.
When food burns on a stove, it changes color and becomes a new substance with different properties. This is an example of a chemical change. Dry ice sublimates and becomes a gas. It is a physical change as the form of dry ice changes, but it does not become a different substance.
For more such questions on physical and chemical changes, click on:
https://brainly.com/question/11370755
#SPJ11
zn2 (aq) solution is electrolyzed using a current of 0.60 amps. what mass of zn(s) is plated out after 5 hours?
Answers
The mass of Zn(s) plated out after 5 hours is approximately 3.656 grams.
To determine the mass of Zn(s) plated out after 5 hours of electrolyzing a Zn2+(aq) solution with a current of 0.60 amps, follow these steps:
1. Calculate the total charge passed through the solution using the formula: charge (Q) = current (I) x time (t).
Q = 0.60 A x (5 hours x 3600 seconds/hour) = 0.60 A x 18000 s = 10800 C (Coulombs)
2. Determine the number of moles of electrons using Faraday's constant (F = 96485 C/mol):
moles of electrons = Q / F = 10800 C / 96485 C/mol = 0.1119 mol
3. Calculate the number of moles of Zn2⁺ reduced to Zn(s) using the stoichiometry of the redox reaction (2 electrons per 1 mole of Zn2⁺):
moles of Zn2+ = 0.1119 mol electrons / 2 = 0.05595 mol Zn2⁺
4. Calculate the mass of Zn(s) using the molar mass of Zn (65.38 g/mol):
mass of Zn(s) = 0.05595 mol Zn2⁺ x 65.38 g/mol = 3.656 g
Therefore, The mass of Zn(s) plated out after 5 hours is approximately 3.656 grams.
To know more about stoichiometry, visit:
https://brainly.com/question/30215297
#SPJ11
How many total electrons can the p orbitals hold?

Answers
Answer:
6
Explanation:
Answer: 6
Explanation:
I just took this thang
Do you feel that this equation is currently following the law of conservation of mass?
N2 + H2 → NH
Answers
Answer:
N2 + H2 --> 2 NH
Explanation:
You need to add 2 to make it correct
Chemistry 1 question please help!! 10 points
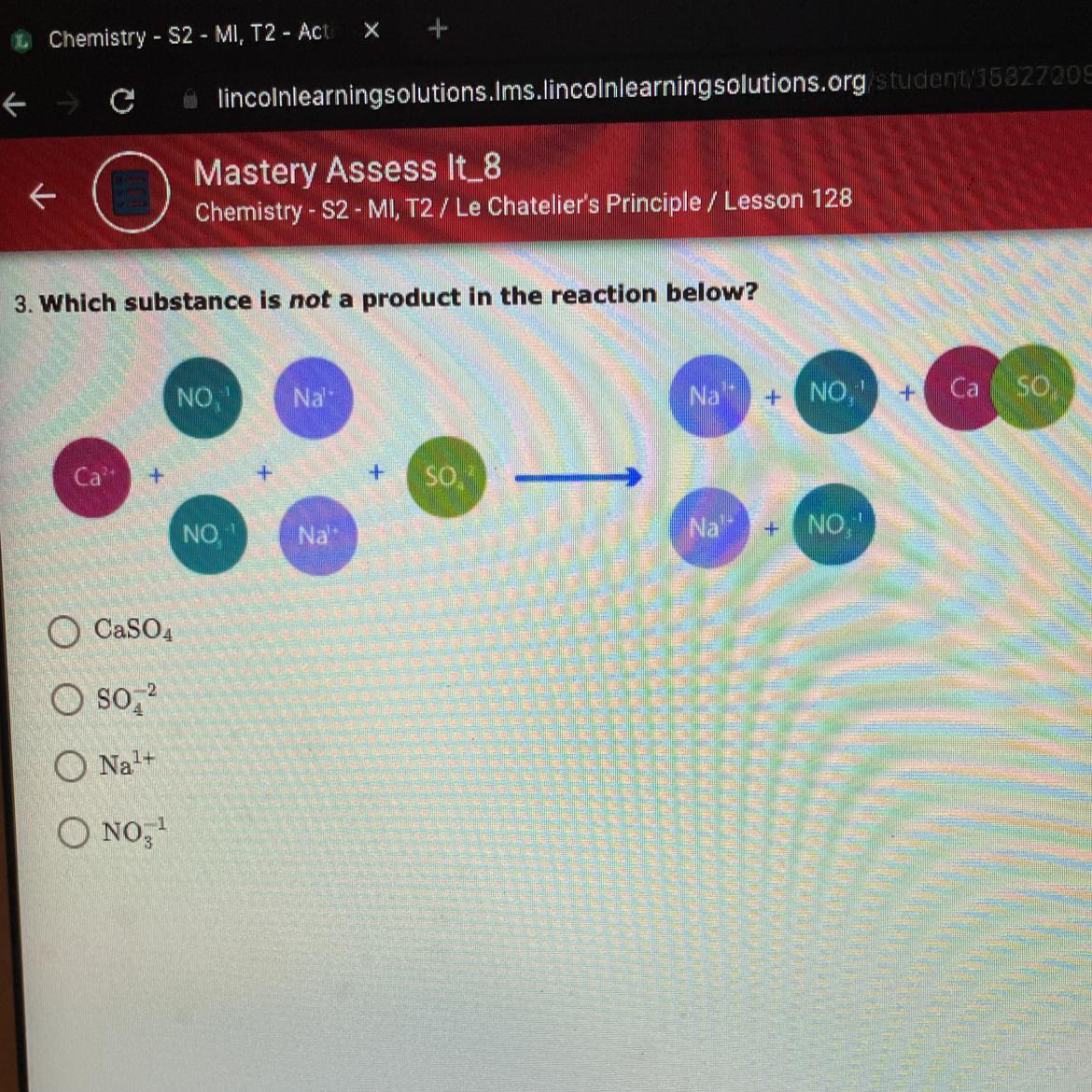
Answers
Answer:
3
Explanation:
its the 3 one it is