Question Which of the hypothesis tests listed below is a left-tailed test? Select all correct answers. Select all that apply OH 18, H₁ 19.3 H₂8. H:/8. OH-11.3 Hp
Answers
Among the given options, the tests that are left-tailed are H0:μ≥18, Ha:μ<18, H0:μ≥11.3, Ha:μ<11.3, and H0:μ≥3.7, Ha:μ<3.7.
In these tests, the null hypothesis (H0) states that the population mean (μ) is greater than or equal to a specific value, while the alternative hypothesis (Ha) suggests that the population mean is less than that value.
A left-tailed test is used when the alternative hypothesis suggests that the population parameter is less than a certain value.
This indicates a left-tailed test, where the critical region is in the left tail of the distribution. These tests focus on detecting a significant decrease or difference in the population mean.
To know more about the left-tailed test refer here :
https://brainly.com/question/31431592#
#SPJ11
Complete question :
Which of the hypothesis tests listed below is a left-tailed test? Select all correct answers.
Select all that apply: H0:μ≥18, Ha:μ<18 H0:μ≤19.3, Ha:μ>19.3 H0:μ=8, Ha:μ≠8 H0:μ≥11.3, Ha:μ<11.3 H0:μ≥3.7, Ha:μ<3.7
Related Questions
Explain how each of the following tools help scientists observe things.
Help me with the first one

Answers
Which of the following statements concerning mixtures is correct?
a. The composition of a homogeneous mixture cannot vary.
b. A homogeneous mixture can have components present in two physical states.
c. A heterogeneous mixture containing only one phase is an impossibility
d. More than one correct response..
Answers
The correct option from the given statements concerning mixtures is (d) more than one correct response.
The statement (a) "The composition of a homogeneous mixture cannot vary" is incorrect as the composition of a homogeneous mixture can vary. For example, a mixture of salt and water is homogeneous and its composition can vary depending on the amount of salt and water mixed in it.
The statement (b) "A homogeneous mixture can have components present in two physical states" is correct. Homogeneous mixtures are mixtures that are uniform throughout their composition, meaning that there is no visible difference between the components of the mixture. For example, a mixture of ethanol and water is homogeneous and its components are present in two physical states (liquid and liquid).
The statement (c) "A heterogeneous mixture containing only one phase is an impossibility" is incorrect. A heterogeneous mixture is a mixture where the components are not evenly distributed and the mixture has different visible regions or phases. However, it is possible for a heterogeneous mixture to contain only one phase. For example, a mixture of oil and water is heterogeneous but can have only one phase.
To know more about homogeneous visit:
https://brainly.com/question/30587533
#SPJ11
Calculate the volume in L of 11.6 moles of Neon at 120 K when it has a pressure of 25.9 atm
Answers
Answer:
The volume of the gas is approximately 4.41 liters
Explanation:
The details of the data of the Neon gas are;
The number of moles of Neon gas present, n = 11.6 moles
The temperature of the sample of Neon gas, T = 120 K
The pressure of the sample of the Neon gas, P = 25.6 atm
By the ideal gas equation, we have;
P·V = n·R·T
Where;
R = The universal gal constant = 0.08205 L·atm·mol⁻¹·K⁻¹
Therefore, we get;
V = n·R·T/P
Which gives;
V = 11.6 moles × 0.08205 L·atm·mol⁻¹·K⁻¹ × 120 K/(25.9 atm) ≈ 4.4097915 L
The volume of the gas, V ≈ 4.41 L.
A solution of 50% dextrose 500 mL, 8.5% Aminosyn 500 mL, and sterile water for injection 300 mL is ordered. What is the total weight (in grams) of the dextrose? ANS: - What is the total weight (in grams) of Aminosyn? ANS: -20 mEq of KCI are needed in the infusion above. How many mL of KCI should be added? Stock strength available: KCl 2 mEq/mL ANS: 22 mEq of NaCl are also needed in the infusion. What volume of NaCl should be added? Stock strength available: NaCl 4.4 mEq/mL ANS: What is the total volume of solution with the original fluids and the addition of KCI and NaCI? ANS:
Answers
The total weight of dextrose is 250 grams.
The total weight of Aminosyn is 42.5 grams.
10 mL of KCI should be added.
5 mL of NaCl should be added.
The total volume of the solution with the original fluids and the addition of KCI and NaCl is 1315 mL.
To calculate the total weight of dextrose, we need to know the concentration of the 50% dextrose solution. Assuming the concentration refers to weight/volume (w/v), we can calculate the weight using the formula:
Weight of dextrose = (Concentration of dextrose * Volume of dextrose solution) / 100
Weight of dextrose = (50 * 500) / 100
Weight of dextrose = 250 g
Therefore, the total weight of dextrose is 250 grams.
To calculate the total weight of Aminosyn, we need to know the concentration of the 8.5% Aminosyn solution. Assuming the concentration refers to weight/volume (w/v), we can calculate the weight using the same formula as above:
Weight of Aminosyn = (Concentration of Aminosyn * Volume of Aminosyn solution) / 100
Weight of Aminosyn = (8.5 * 500) / 100
Weight of Aminosyn = 42.5 g
Therefore, the total weight of Aminosyn is 42.5 grams.
To calculate the volume of KCI to be added, we need to know the strength of the stock KCI solution. Assuming the stock KCI solution is 2 mEq/mL, we can calculate the volume of KCI using the formula:
Volume of KCI = (Amount of KCI needed) / (Strength of KCI solution)
Volume of KCI = 20 mEq / 2 mEq/mL
Volume of KCI = 10 mL
Therefore, 10 mL of KCI should be added.
To calculate the volume of NaCl to be added, we need to know the strength of the stock NaCl solution. Assuming the stock NaCl solution is 4.4 mEq/mL, we can calculate the volume of NaCl using the formula:
Volume of NaCl = (Amount of NaCl needed) / (Strength of NaCl solution)
Volume of NaCl = 22 mEq / 4.4 mEq/mL
Volume of NaCl = 5 mL
Therefore, 5 mL of NaCl should be added.
The total volume of the solution with the original fluids and the addition of KCI and NaCl can be calculated by adding the volumes of all the components:
Total volume = Volume of dextrose solution + Volume of Aminosyn solution + Volume of sterile water + Volume of KCI + Volume of NaCl
Total volume = 500 mL + 500 mL + 300 mL + 10 mL + 5 mL
Total volume = 1315 mL
Therefore, the total volume of the solution is 1315 mL.
Learn more about Volume from the link given below.
https://brainly.com/question/28058531
#SPJ4
mix 3 liters of water at 40 oc with 1 liter of water at 20 oc and the final temperature is group of answer choices more than 30oc less than 20 oc less than 30oc about 30oc
Answers
The final temperature is approximately 35°C. So, the correct answer is (1).
Let's assume the final temperature is T°C.
For the 3 liters of water at 40°C:
Heat gained = mass × specific heat capacity × temperature change
Heat gained = (3 kg) × (4.186 J/g°C) × (T - 40)
For the 1 liter of water at 20°C:
Heat lost = (1 kg) × (4.186 J/g°C) × (20 - T)
Since the total heat gained and lost are equal:
(3 kg) × (4.186 J/g°C) × (T - 40) = (1 kg) × (4.186 J/g°C) × (20 - T)
Simplifying the equation, we find:
\(3(T - 40) = 20 - T\\3T - 120 = 20 - T\\4T = 140\)
T = 35°C
The correct answer is (1).
To know more about specific heat capacity, here
brainly.com/question/1453843
#SPJ4
--The complete question is, mix 3 liters of water at 40 oc with 1 liter of water at 20 oc and the final temperature is group of answer choices
1.more than 30oc
2.less than 20 oc
3.less than 30oc
4.about 30oc--
How many liters of wine can be held in a wine barrel whose capacity is 30.0 gal?
Answers
1 gallon is approximately 3.78541 liters, so to convert to liter, we can multiply by this number:
\(30.0gal=30.0\cdot3.78541L=113.562L\approx114L\)So, 30.0 gal is approximately 114L.
Question 3 3 pts The indicator used in this experiment is Calmagite, and to ensure we can monitor the color change clearly, the pH of the calcium solution needs to be adjusted to Select | using a pH buffer solution. In this experiment, when Calmagite binds to the metal ions in the water sample, the solution before EDTA titration will exhibit a color of Select and the solution after the EDTA titration will exhibit a color of Select
Answers
The change in color indicates the endpoint of the reaction, which can be used to determine the concentration of calcium in water.
The indicator used in this experiment is Calmagite, and to ensure that the color change is clearly monitored, the pH of the calcium solution needs to be adjusted to pH 10 using a pH buffer solution. In this experiment, when Calmagite binds to the metal ions in the water sample, the solution before EDTA titration will exhibit a color of purple, and the solution after the EDTA titration will exhibit a color of blue.How to determine calcium in water?The calcium in water can be determined by titration, which is a technique that is used to determine the concentration of a substance in a solution. EDTA titration is used to determine the concentration of calcium in water. The process involves the addition of EDTA (ethylenediaminetetraacetic acid) to the sample, which forms a complex with calcium ions. EDTA is a chelating agent that can bind to metal ions such as calcium, and in the presence of a suitable indicator, the endpoint of the reaction can be detected.A suitable indicator for this reaction is Calmagite, which is a purple-colored compound that changes color to blue when it binds to calcium ions. The pH of the solution needs to be adjusted to pH 10 using a pH buffer solution to ensure that the color change is clearly monitored. The solution before EDTA titration will exhibit a color of purple, and the solution after the EDTA titration will exhibit a color of blue. The change in color indicates the endpoint of the reaction, which can be used to determine the concentration of calcium in water.
Learn more about Calmagite here,
https://brainly.com/question/30559544
#SPJ11
* I’ll give you brainlist* The skeletal system has many functions. Which of the following body functions does the skeletal system NOT do?

Answers
Answer:
A) remove germs from the blood
Explanation:
cause the rest are the skeletal system function
Answer:
The skeletal system does not remove germs from the blood
Explanation:
I learned that from my teacher
When the umbilical cord is tied after birth, the umbilical arteries close by filling in with. A) placental fluid. B) platelet plugs. C) connective tissue.
Answers
When the umbilical cord is tied after birth, the umbilical arteries close by filling in with connective tissue. The umbilical arteries carry deoxygenated blood from the fetus to the placenta, where the blood is oxygenated and returned to the fetus via the umbilical vein.
When the umbilical cord is cut, the flow of blood from the placenta to the fetus ceases, and the umbilical arteries and vein begin to constrict. This constriction is caused by the contraction of smooth muscles in the vessel walls and the closure of small valves within the vessels. As the umbilical arteries constrict, the flow of blood to the placenta decreases and the vessels begin to fill in with connective tissue. Over time, the connective tissue replaces the smooth muscle and valve tissue in the vessel walls, resulting in the complete closure of the umbilical arteries. This process is important to prevent bleeding and infection in the newborn.
Learn more about placenta here:
https://brainly.com/question/26959441
#SPJ11
Which method can be used to calculate the percentage composition of a
compound?
Answers
I uploaded the answer to a file hosting. Here's link:
tinyurl.com/wpazsebu
If a right triangle has sides 13 and 5, what is the missing number
Answers
The missing number in the right triangle with sides 13 and 5 can be found using the Pythagorean theorem, which states that the sum of the squares of the legs (the two shorter sides) is equal to the square of the hypotenuse the longest side.
So, the main answer is that the missing number is the length of the hypotenuse. To find it, we can use the pythagorean This is the long answer and the exact value of the hypotenuse in simplified radical form. However, if we want a decimal approximation.
In a right triangle, the Pythagorean theorem applies, which states that the square of the length of the hypotenuse (the side opposite the right angle) is equal to the sum of the squares of the lengths of the other two sides.
In this case, you are given two sides: 13 and 5. One of them must be the hypotenuse.
Since 13 > 5, 13 is the hypotenuse.
To know more about hypotenuse Visit;
https://brainly.com/question/29730442
#SPJ11
What process is used to refine aluminum from its ore bauxite?
A. Electrolysis
B. Electrolytic oxidation
C. Voltaic cell processing
D. Smelting
Answers
The correct option for the process used to refine aluminum from its bauxite ore is option D.
D. Smelting
The reason why smelting is the correct choice is as follows;
Bauxite is the aluminum ore from which alumina, also known as aluminum
oxide, Al₂O₃, is produced. Bauxite is extracted from the topsoil regions of
some subtropical and tropical regions, and the Bayer process is primarily
then used to produce alumina from the bauxite.
Aluminum is produced from the alumina by an aluminum smelting process
known as the Hall—Heroult electrolytic process which involves the use of a
carbon anode and direct current to produce aluminum by reducing the
aluminum oxide
Learn more about smelting process here:
https://brainly.com/question/3444206
3
1. The observed regularities in the properties of
the elements are periodic functions of their
(1) atomic numbers
(2) mass numbers
(3) oxidation states
(4) nonvalence electrons
Answers
Answer:
(1) atomic numbers
Explanation:
The observed regularities in the properties of the elements on the periodic table are periodic functions of their atomic numbers.
Atomic number is the number of protons in an atom. The periodic law states that "the properties of elements are a periodic function of their atomic number".Elements on the periodic table are arranged based on the atomic numbers they contain. The number of positively charged particles in an atom is the atomic number.The observed regularities in the properties of the elements are periodic functions of their atomic numbers.
Modern periodic law states that "Properties of elements are a periodic function of their atomic number not by their atomic mass". Modern periodic law was proposed by Henry Moseley in 1913. Physical and chemical properties of elements depend on the number of electrons and their arrangement.
Thus properties of elements are periodic function of their atomic numbers and not atomic masses. If the properties of an element depend on number of proton and neutron then the properties are periodic function of their atomic mass so we can conclude that the observed regularities in the properties of the elements are periodic functions of their atomic numbers.
Learn more: https://brainly.com/question/19534915
Li+1 had gained ______ one electron
Answers
What happens to iron in a bolt as the bolt rusts?
Answers
Answer:
write the following as fractions in their simplest form
H. If you had 64 amu of ch4, how many molecules would this be? i. If you had 64 g of ch4, how many moles would this be?
Answers
The number of molecules and number of moles of \(CH_{4}\) is 4 moles and 24.088×\(10^{23}\) molecules.
Calculation,
Atomic mass of carbon is 12 u and H is 1 u
So, molar mass of \(CH_{4}\) = 12 + 1×4 = 16 u = 16 g/mole
Number of moles = given mass / molar mass = 64 g/ 16 g/mol = 4 moles
1 moles of substance contains 6 .022 ×\(10^{23}\) molecules.
So, number of molecules in 3 moles of methane = 4×6.022 ×\(10^{23}\)
number of molecules in 3 moles of methane = 24.088×\(10^{23}\) molecules.
learn about moles
https://brainly.com/question/26416088
#SPJ4
In lab, you may need to evaluate the odor or smell of a chemical. What is the best way to smell a chemical sample?.
Answers
Given what we know about lab safety, we can confirm that the best way to smell a chemical sample when needed, is to place the sample under your nose at a safe distance and smell very slowly.
Why is this the best way to smell the sample?Many chemicals can at times emit relatively toxic fumes. These fumes can be very dangerous to inhale. Therefore, it is best to take safety precautions when attempting to smell a sample. Placing the sample at a distance and smelling slowly is a good habit as it gives you time to react to an unpleasant or potentially dangerous smell.
Therefore, we can confirm that since many chemicals in a lab can be dangerous and can often have relatively toxic fumes, extreme care should be taken if attempting to smell them. Placing the sample at a safe distance and smelling slowly is a good safety precaution to take.
To learn more about lab safety visit:
https://brainly.com/question/20103808?referrer=searchResults
Q:- Calculate the number of ions of the following compound:- 16g of H₂CO3
Answers
Answer:
= 1.553 x 10²³ ions
Explanation:
One mole of a compound or a substance consists of 6.02 x 10²³ ions. 6.02 x 10²³ is also referred to as Avogadro's number/constant.
From the above question, we have been asked to determine the number of ions in 16g of H₂CO3.
The relative formula mass (RFM) of H₂CO3 is 62.026 u:
Derived as;
H₂CO3 = (2 x 1.008) + (12.01 x 1) + (3 x 16.00) u
= 62.026 u.
Number of moles = Mass/RFM
\(moles \: = \frac{16}{62.026} \\ = 0.2579563 \\ = 0.2580 \: moles\)
1 mole = 6.02 x 10²³ ions
0.2580 moles = ?
=
\( = \frac{0.2580 \times 6.02 \times 10 ^{23} }{1} \\ = 1.553 \times {10}^{23} \: ions\)
Hence the number of ions in 16g H2CO3 = 1.553 x 10²³
An aqueous solution is 10.% glucose by mass (d = 1.039 g/mL at 20°C). Calculate its freezing point, boiling point at 1 atm, and osmotic pressure
Answers
The values of
Boiling point for the solution: 100.32°C
Freezing point for the solution: -1.15°C
Osmotic pressure for the solution at 20°C: 13.9 atm
What is osmotic pressure?The osmotic pressure is the least amount of pressure that must be applied to a solution in order to block the passage of the solution's pure solvent through a semipermeable membrane.It can alternatively be described as a measurement of a solution's propensity to osmotically absorb a pure solvent. The highest osmotic pressure that may form in a solution if it were cut off from its pure solvent by a semipermeable membrane is known as the potential osmotic pressure.When two solutions with various solute concentrations are separated by a selectively permeable membrane, osmosis takes place.From the low-concentration solution to the solution with increasing solute concentration, solvent molecules move across the membrane preferentially. Up until equilibrium is reached, solvent molecules will continue to move.we know that
Mass = \(volume \times density\)
Mass = \(1.039 \times 1000 = 1039\)gm solution
Then the amount of the solute and solvent are
Mass = \(0.10 \times 1039 = 103.9 g\) solute
Mass = \(0.9 \times 1039 = 935.1g\) solvent
now moles of glucose = \(\frac{103.9}{180} = 0.577 moles\)
now molarity M = \(\dfrac{moles}{volume} = \dfrac{0.577}{1} = 0.577\)
we know that
\(\Delta T = K_b \times m = 0.577 \times 0.617 = 0.32\)
hence the boiling point is
\(\Delta T = T_b - T\\T_b = 100 + 0.32 = 100.32\)
osmotic pressure = \(\pi = MRT\\\pi = 0.577 \times 0.082 \times 293 =13.9 atm\)
To learn more about osmotic pressure with the given link
https://brainly.com/question/12497098
#SPJ4
I need help with number 5 pleaseee
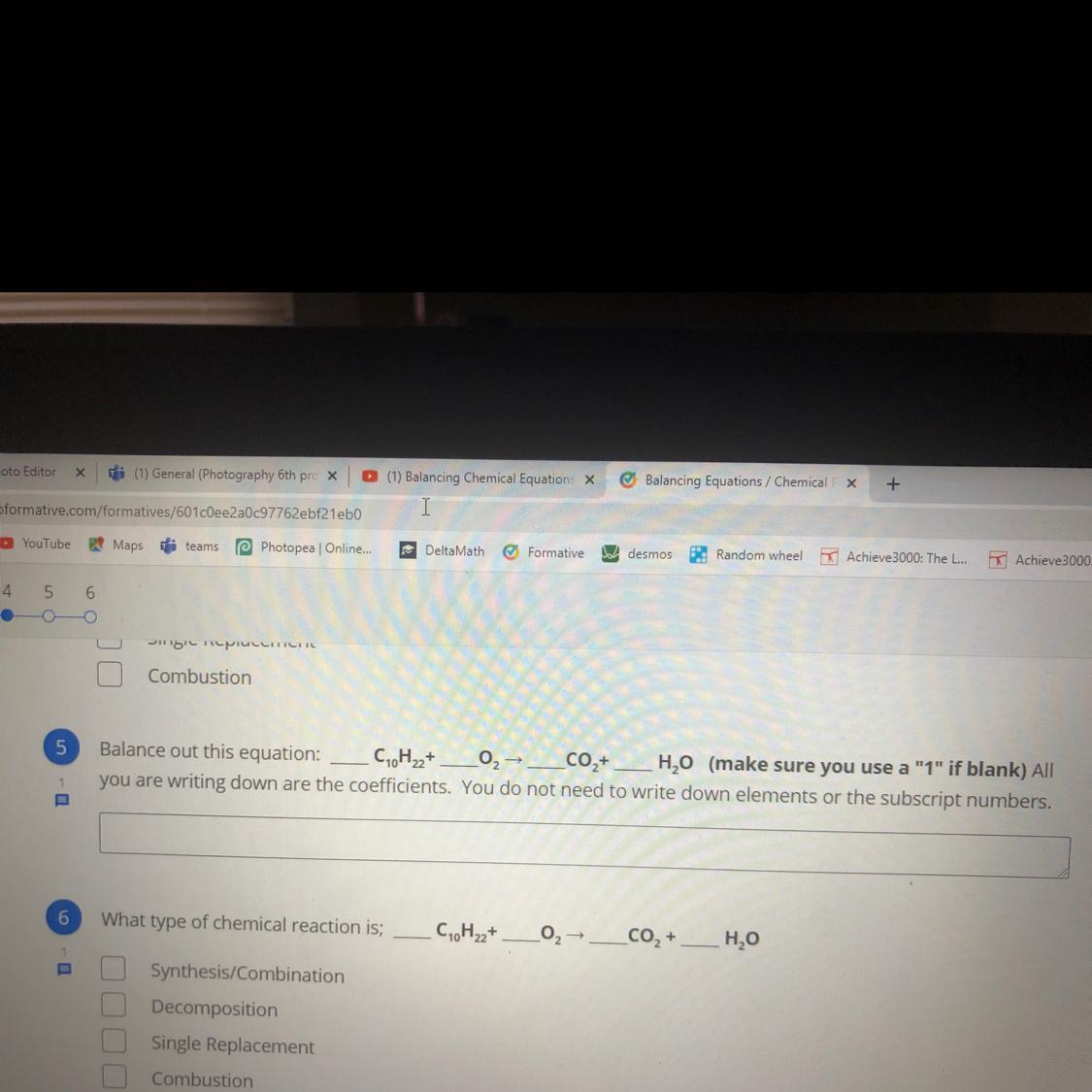
Answers
Answer: the answer is d
Explanation:
Decane + Dioxygen = Carbon Dioxide + Water
Reaction Type
Combustion
Compare the life cycles of a flowering and a conifer non-flowering plant.
Answers
Answer:
The main difference between flowering and nonflowering plants is their method of reproduction. Flowering plants rely on pollination for reproduction, where as nonflowering plants rely on dispersion to continue their life cycle.
Of the following, which can be used to identify an element present in a sample?
Answers
Answer:
idk but i tryed
Explanation:
The simplest way to use the periodic table to identify an element is by looking for the element’s name or elemental symbol. The periodic table can be used to identify an element by looking for the element’s atomic number. The atomic number of an element is the number of protons found within the atoms of that element.
The reaction of hydrogen bromide(g) with chlorine(g) to form hydrogen chloride(g) and bromine(g) proceeds as follows:
2HBr(g) + Cl2(g) -->2HCl(g) + Br2(g)
When 23.5 g HBr(g) reacts with sufficient Cl2(g), 11.8 kJ is evolved.
Calculate the value of △,H for the chemical equation given.
Answers
The enthalpy of reaction per mole of HBr for this
reaction = ArH =-40.62 kJ/mole.
Explanation:
2HBr(g) + C12(g) > 2HC|(g) + Br2 (g)
When 23.9 g HBr(g) reacts with sufficient C12(g),
12.0 kJ of heat is evolved, calculate the value of
Ar for the chemical reaction.
Note that ArH is the enthalpy per mole for the
reaction.
Molar mass of HBr (g) = 80.91 g/mol.
Hence, 1 mole of HBr = 80.91 g
23.9 g of HBr led to the reaction giving off 12.0
kJ of heat
80.91 g of HBr will lead to the evolution of (80.91
× 12/23.9) = 40.62 kJ heat is given off.
Hence, 40.62 kJ of heat is given off per 80.91 g
of HBr.
This directly translates to that 40.62 kJ of heat is
given off per 1 mole of HBr
Hence, the heat given off per mole of HBr for
this reaction is 40.62 kJ/mole.
But since the reaction liberates heat, it means
the reaction is exothermic and the enthalpy
change for the reaction (AHrxn) is negative.
-40.62
Someone pls tell me the answer

Answers
Answer:
6 inches and that should be the answer
describe the relationship between pressure and temperature
Answers
It is used in the design of engines, where changes in pressure and temperature are used to convert thermal energy into mechanical work. It is also used in meteorology to predict weather patterns and in the study of the Earth's atmosphere.
Pressure and temperature are two fundamental physical quantities that are closely related in many physical processes. Understanding the relationship between these two quantities is essential in many scientific and engineering fields. This relationship can be described by the ideal gas law, which states that the pressure of an ideal gas is directly proportional to its temperature when the volume and number of particles are constant. In other words, when the temperature of a gas increases, its pressure increases, and vice versa. This can be explained by the kinetic theory of gases, which assumes that gases are made up of a large number of small particles that are in constant motion. The speed of these particles is proportional to the temperature of the gas. As the temperature of the gas increases, the particles move faster and collide more frequently with the walls of the container, resulting in an increase in pressure. Similarly, when the temperature of the gas decreases, the particles move slower and collide less frequently with the walls of the container, resulting in a decrease in pressure. This relationship between pressure and temperature is essential in many scientific and engineering applications.
for more questions on meteorology
https://brainly.com/question/16565664
#SPJ8
Which of the following is NOT a sign of a chemical reaction?
A. Change in color
B. Change in size
C. Bubble formation
D. A temperature change
Answers
Diazomethane is a toxic yellow gas that is both sensitive and explosive: However, it is a useful reagent in the laboratory to make key intermediates by reacting with carboxylic acids in a quick and clean reaction_ Draw the expected organic product of diazomethane with the following carboxylic acid. COOH Hc N=N; CH3
Answers
Methyl esters are created when carboxylic acids and diazomethane interact. Diazomethane is used to create methyl esters and has a high degree of reactivity.
Diazomethane reacts swiftly and extremely effectively, producing just N2 as a byproduct, making it an appealing alkylating agent for carboxylic and phenols (Black, 1983). As it responds, its inherent yellow color releases, automatically indicating the status of the reaction. Methyl esters are created when carboxylic acids and diazomethane interact. Diazomethane is created in-situ and then interacted with the carboxyl group right away to create the methyl ester due to its high reactivity. Diazomethane has mostly been used to transform carboxylic acids into esters (diazoalkales) that may be examined using GC/MS or HPLC-MS. In etherate solutions, methyl esters can be produced at room temperature quickly, thoroughly, and quantitatively.
Learn more about acids
https://brainly.com/question/29426933
#SPJ4
In one to two sentences, explain why there is more concern about pollutants in the air during cold months
Answers
Air pollution worsens in winter as cold and dry air contains more pollutants. This makes it easy for pollutant air near the surface of the earth to rise and be lifted away.
As a result of the presence of compounds in the atmosphere that are hazardous to human health and the health of other living things, or that impair the climate or materials, air pollution is the contamination of the air.
It is known that cold air usually sinks and warm air usually rises. Most of the time, temperature decreases with elevation. This makes it easy for pollutant air near the surface of the earth to rise and be lifted away.
However, during winter, thermal inversions are more likely to happen. Because sunlight is weaker during this season, air near the earth’s surface may end up being cooler than the air above, causing the pollution-filled air near to earth's surface. Hence, Colder air traps more pollution.
It is also true that rain can wash away pollutants. But in the dry winter season, due to less humidity in the air, the chances of rainfall are generally lower. Hence, dry air contains more pollutants. Therefore, air pollution is worse in winter.
Learn more about air pollution here: https://brainly.com/question/15158482
#SPJ4
given c22-, using molecular orbital and valence bond theory; 1. write molecular orbital configuration 2. determine bond order and indicate stability 3. identify the magnetic properties (paramagnetic or diamagnetic)
Answers
Bond order and indicate stability is 3 and magnetic property is diamagnetic (no unpaired electron in MO diagram).
1. MO Configuration for \(C2^2\)-
\((14e^-): \sigma^2 1s \sigma^{*2}1s\sigma^2 2s \sigma^{*2}2s\pi^2 2p \pi^{2} 2p \pi^{2}\sigma^22p\)
2. Bond order = (8-2)/2 = 3
3. Magnetic property = diamagnetic (no unpaired electron in MO diagram).
Bond order refers back to the number of chemical bonds among a pair of atoms. It is a measure of the strength and stability of a chemical bond. Bond order is typically calculated by taking the difference between the number of bonding electrons and the number of antibonding electrons in a molecule, and dividing that number by two.
Bond order is an important concept in chemistry as it provides insight into the chemical properties of a molecule, such as its reactivity and stability. It can also help predict the bond length and bond energy of a molecule, which are important factors in understanding how molecules interact and react with one another.
To learn more about Bond order visit here:
brainly.com/question/12447843
#SPJ4
Water is being lost from earth at the rate per a year of what percentage
Answers
At least 14 to 18 percent of water is being lost each year. I really hope this helps :D
Answer:
The current loss figure is equivalent to ~25,920 liters per day or 9,467 m3 per year
It isn't a percentage, but I hope that helps a little