Sandy wanted to find out if the color of food would affect..
Answers
Related Questions
What is the average of atomic mass of carbon if 98.90% of the atoms are c-12 and 1.100% are c-13 atoms?
Answers
Answer:
special reform undertaken difficult socio P think Dubai terrorism graphi tachyon duck futuro custom symbol waveform Washington zeaxanthin decal celestial fig
The modern periodic table is arranged in order of increasing atomic
would it be atomic number?
Answers
The modern periodic table of elements is arranged in the increasing order of atomic number.
What is the modern periodic table?The Modern Periodic Table is the arrangement of the elements in the order of their atomic numbers in seven horizontal rows called periods and eighteen vertical columns known as groups.
The modern periodic table is based on the Modern Periodic Law which states that the chemical and physical properties of the elements are the functions of atomic numbers.
The elements present in the same group have the same valence electronic configuration. So, their chemical properties are similar.
Therefore, the elements in the modern periodic table are arranged in an ascending order of their atomic numbers.
To learn more about modern periodic table, here:
https://brainly.com/question/14263041
#SPJ1
Find the pH of a 3.50 x 10^-4 M H2SO4 solution (sulfuric acid).

Answers
Answer:
3.46
Explanation:
-log(3.50*10^-4) = 3.46
2. How many calories of heat are required to raise the temperature of 225g of
water from 10.5°C to 43.7°C7 QmCAT (Cate 1,00cal/g C)
Answers
It requires 7458 calories of heat to raise the temperature of 225 grams of water from 10.5°C to 43.7°C.
To calculate the amount of heat required to raise the temperature of a substance, we can use the formula Q = m * C * ΔT, where Q represents the heat, m is the mass of the substance, C is the specific heat capacity, and ΔT is the change in temperature.
In this case, we have 225 grams of water, a specific heat capacity of 1.00 cal/g°C, and a temperature change of 33.2°C (from 10.5°C to 43.7°C).
Plugging these values into the formula:
Q = 225 g * 1.00 cal/g°C * 33.2°C
Q = 7458 cal
Therefore, it requires 7458 calories of heat to raise the temperature of 225 grams of water from 10.5°C to 43.7°C.
This calculation is based on the specific heat capacity of water, which is the amount of heat energy required to raise the temperature of water by 1°C per gram. The specific heat capacity of water is relatively high compared to other substances, which is why it takes a significant amount of heat to raise its temperature.
It's important to note that the specific heat capacity of water can vary slightly with temperature, but for practical purposes, we often assume a constant value of 1.00 cal/g°C.
By using the given values and the formula for heat, we can accurately determine the amount of heat required for this specific temperature change in the given mass of water.
for more such questions on temperature
https://brainly.com/question/4735135
#SPJ11
Which event would most likely cause an ecosystem to have the lowest biodiversity and population sizes 3 years later?
O A lava flow creates a new section of land
O A forest fire destroys the plant growth
O A river floods a cornfield
O Clearing land for a highway
Answers
Answer:
Clearing Land for a highway
Explanation:
The other examples are that of naturally occurring disasters and most life in that region have ways to regrow and repopulate in the case of those disasters. While a highway is a manmade object and interrupts the environment to the point that the new animals will not know how to deal with it. That is also why there are a lot of dead deer are found on the side of the road when a new highway is built.
Clearing land for a highway would most likely cause an ecosystem to have the lowest biodiversity and population sizes 3 years later. Therefore, option D is correct.
What is biodiversity ?Biodiversity refers to the variety of life that can be found in a given area, including animals, plants, fungi, and even microorganisms such as bacteria. Each of these species and organisms collaborate in ecosystems to maintain balance and support life, much like an intricate web.
Every one of these exists in delicate balance and lives and works together in ecosystems to sustain and support life on Earth.
Typically, three levels of biodiversity are discussed: genetic diversity, species diversity, and ecosystem diversity. Genetic diversity refers to the various genes found in all plants, animals, fungi, and microorganisms. It can occur both within and between species.
Thus, option D is correct.
To learn more about the biodiversity, follow the link;
https://brainly.com/question/13073382
#SPJ6
Why cant you just add up the average masses, divide by three and get an atomuc mass?
Answers
Answer:
Differences are particle, nor does it measure their total mass as you did. particles and their relative abundances. It does not count each individual A mass spectrometer yields only information about the masses of atomic They are equal.
Explanation:
HOPE IT HELPS
Name:
1. The nucleus is the part of the atom that
A consist mostly of empty space
B.
has a negative charge
Coccupies most of the atom's total volume
D. contains most of the atom's total mass
Answers
Answer:
D.
Explanation:
There are three types of particles within atoms: protons, neutrons, and electrons.
Electrons have essentially no mass and orbit the nucleus in various energy levels.
Protons and neutrons have mass and are both found within the nucleus of the atom.
which two ions are you most likely to see adsorbed to the exchange sites of a soil in an arid environment?
Answers
The two ions most likely to be adsorbed to the exchange sites of a soil in an arid environment are calcium (Ca2+) and magnesium (Mg2+).
In arid environments, the soil tends to have higher levels of alkaline earth metals, such as calcium and magnesium. These ions are often present in the soil solution and can be adsorbed to the negatively charged exchange sites on soil particles.
The process of adsorption occurs due to the attractive forces between the positively charged ions and the negatively charged exchange sites. Calcium and magnesium ions, being divalent cations, have a higher charge density and can form stronger electrostatic interactions with the soil surface compared to monovalent cations like sodium or potassium. Therefore, they are more likely to be adsorbed and retained by the soil.
The adsorption of calcium and magnesium to soil exchange sites can have significant effects on soil fertility and nutrient availability. These ions can displace other cations from the exchange sites and influence the overall soil nutrient balance. Additionally, the presence of high levels of calcium and magnesium in arid soils can contribute to soil alkalinity.
It's important to note that the specific composition of ions adsorbed to soil exchange sites can vary depending on factors such as soil type, parent material, and climate. However, in arid environments, calcium and magnesium ions are commonly observed due to their abundance in the soil solution.
Learn more about Soil chemistry
brainly.com/question/32892781
#SPJ11
Farmer brown is planting crops in his feild s. He wants to prevent the topsoil from being blown away by the wind or washed away from by water. Which of these steps should he take. A: plow the soil many times. B: plant crops close together. C: water crops often to wet soil. D: leave the land free of crops for a long time
Answers
I would say the answer is A. Plow the soil many times to lessen the chance of the topsoil being blown away.
Hope this helps !
Answer:
C: water crops often to wet soil.
Explanation:
Working in the plan industry, it becomes obvious that when watering the plants often, it will pack down the topsoil into the plant. Topsoil is lose at first as stated above, but when enough water gets on it, it becomes almost like mud. This is the kind of topsoil you want. No wind or water will mess it up because it already it watered! It will also help the grow. In addition, plowing is not correct because you only need to plow twice in the plant process. Before you plant the seeds, and to harvest the crops. If you plow to soon and often, you won’t have any plants.
Select the correct answer
Giving brainliest

Answers
Answer:
C
Explanation:
You can eliminate A and D because hand warmers are a chemical reaction. And C is the best answer because heat is released.
Having problems figuring out molar mass in general. steps would be most appreciated.
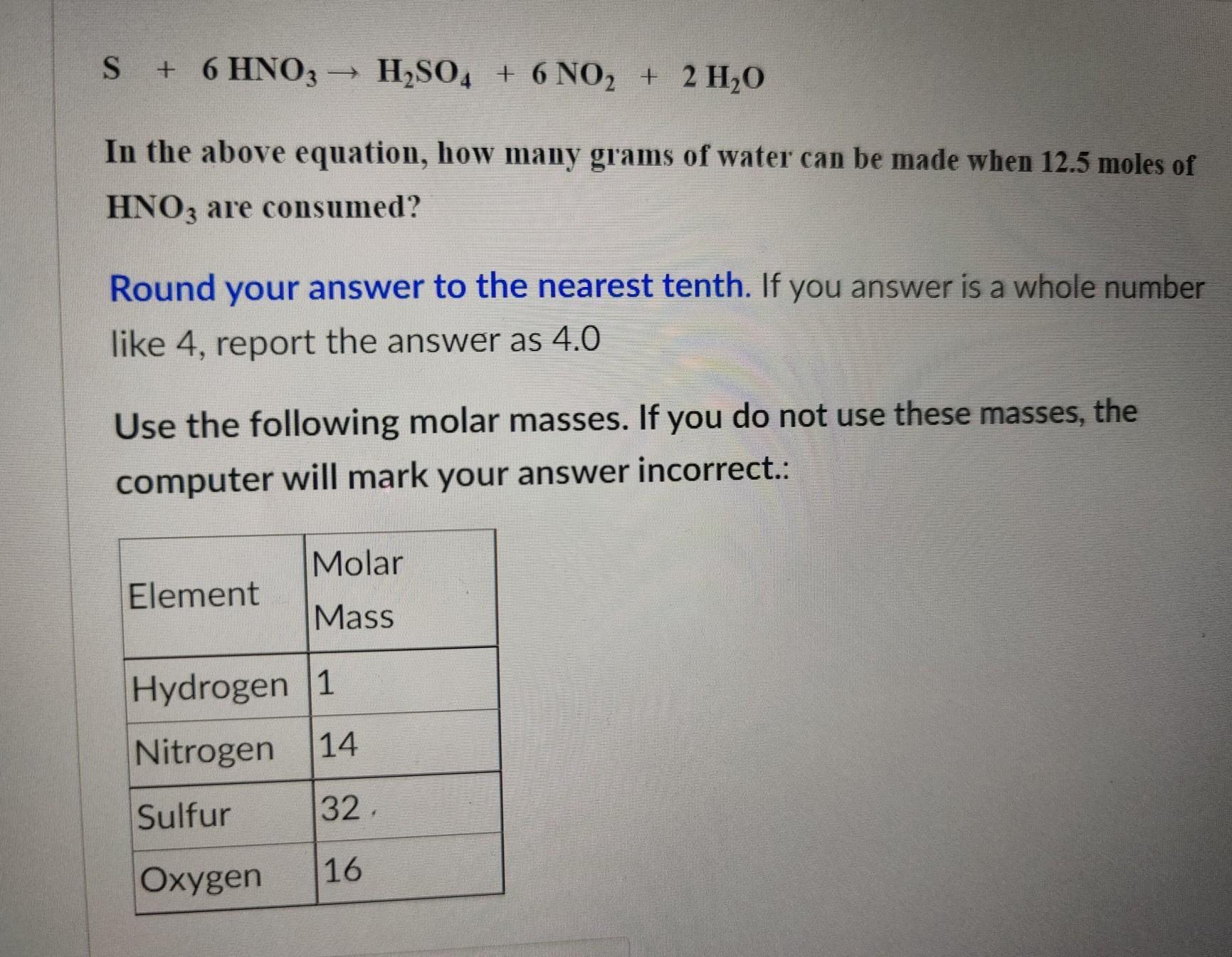
Answers
Answer:
The periodic table
Explanation:
As you can see in the screenshot. Each element on the periodic table has an atomic number and a mass number. The atomic numbers are arranged in numerical order from right to left in the periodic table. The second number there is the atomic mass.
When doing stoichiometric ratios, you need to use the atomic mass of each element to determine the molecular/molar mass.
example. Find the molar mass of
H₂SO₄
Hydrogen (2) + sulphur (1) +oxygen (4)
= atomic mass of H(2)+ atomic mass S(1)+ atomic mass oxygen(4)
=1(2) + 32(1)+16(4)
=2+32+64
=98
N.B We do not use the co-efficient when looking for molar mass, we only use the atom and subscript
3.5 moles of nitric acid
Answers
Answer:
Socratic app
I just learned it
name the ions formed by these elements and classify them as anions or cations. a) selenium b) barium c) phosphorus
Answers
The selenium ion is represented as Se4+, Barium ion is represented as Ba2+ and Phosphorus occurs as phosphate ion i.e., PO43-.
The chemical species that contains a positive charge is known as cation while the chemical species consisting of a negative ion is known as anion.
Selenium is a metal, therefore is exist as a cation with Se4+ representation having +4 charge. Barium is a metal, thus it exist as a cation with Ba2+ representation having +2 charge while phosphorus always occurs as a phosphate ion i.e., PO43- which is an anion as phosphorus is a non-metal.
Therefore, the representation of the ions are given above.
To learn more about ions check the link below:
https://brainly.com/question/13692734
#SPJ4
Which element loses an electron most easily?
F
Cl
P
Na
Answers
The element that is on the left side of the periodic table on the first column, it can lose one in order to become a noble gas.
Calculate the number of atoms contained in 5000cm^3 of carbon dioxide gas at room condition
Answers
Answer:
\(\huge\boxed{\sf No.\ of\ atoms = 1.4 * 10^{20} \ atoms}\)
Explanation:
Given Data:
Volume = v = 5000 cm³ = 0.005 m³
Density of CO₂ at RTP = 1.98 kg / m³
Molar Mass = M = 12 + 16 * 2 = 44 g / mol
Avogadro's No. = \(\sf N_{A}\) = 6.023 * 10²³ mol⁻¹
Required:
Number of atoms = ?
Solution:
We know that:
No. of moles (n) = mass in grams / molar mass ∴ Mass = Density * Volume
n = D * v / M
n = 1.98*0.005 / 44
n = 0.000225
Now, Finding the number of atoms
No. of atoms = No. of moles * Avogadro's Number
No. of atoms = 0.000225 * 6.023 * 10²³
No. of atoms = 0.0014 * 10²³
No. of atoms = 1.4 * 10²⁰ atoms
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807Please help this is due soon

Answers
when solid surfaces slide over each other, the kind of friction that occurs is _ friction
Answers
When solid surfaces slide over each other, the kind of friction that occurs is called Sliding Friction.
How many hours are in 7 months? (assume 30 days in a month)
Answers
Answer:
there is about 5110.01 hours in 7 months
Explanation:
because no 7 months in a row have the same amount of months so about 730.001428571 days in 7 months times the number of months. Hope this helps!
Answer: 730.08 hours x 7= will get you your answer
Explanation: Because, the average month is 30.42 days. A day is 24 hours, so the average month is 730.08 hours ( 30.42 days * 24 hours ). Just do the math above and that should be your answer.
Question 23
Marks: 1
The rate at which atoms of radioactive sources (radionuclides) disintegrate are measured in
Choose one answer.
a. rems
b. rods
c. curies
d. roentgens
Answers
The rate at which atoms of radioactive sources, or radionuclides, disintegrate is measured in curies. A curie is a unit of measure for the amount of radioactive material present. It represents the amount of radioactive material in which 37 billion atoms disintegrate per second.
The disintegration of radionuclides produces ionizing radiation, which can be measured in rems or roentgens.
A rem is a unit of measurement for the amount of ionizing radiation absorbed by living tissue, while a roentgen is a unit of measurement for the amount of ionizing radiation in the air.
In summary, the rate at which atoms of radioactive sources disintegrate is measured in curies, while the amount of ionizing radiation produced by the disintegration can be measured in rems or roentgens. It is important to understand these units of measurement in order to properly monitor and regulate exposure to ionizing radiation, as it can have harmful effects on living organisms.
The rate at which atoms of radioactive sources (radionuclides) disintegrate is measured in curies (c).
To explain further, radioactive sources contain unstable atoms, called radionuclides. These radionuclides undergo disintegration or decay, during which they emit radiation. To quantify this process, we use various units.
Curies (Ci) is a unit of measurement specifically used to express the activity of a radioactive substance, or how quickly atoms in the radioactive source are disintegrating. One curie represents 37 billion disintegrations per second.
It's important to note the other units you mentioned:
- Rems (roentgen equivalent in man) is a unit used to measure the biological impact of ionizing radiation on human tissue.
- Roentgens (R) is a unit used to measure the exposure to ionizing radiation, specifically the amount of radiation that produces a certain amount of ionization in air.
- Rods is not a unit related to radioactivity, but might be confused with control rods, which are used in nuclear reactors to control the rate of nuclear reactions.
In summary, the appropriate unit for measuring the rate at which atoms of radioactive sources disintegrate is curies.
Learn more about radioactive at : brainly.com/question/1770619
#SPJ11
Can someone find from which website this test is?

Answers
Answer:
Try quizlet, they have everything on there
Explanation:
Which property of water contributes most to the ability of fish in lakes to survive very cold winters?.
Answers
Answer:
And that means fish must be able to survive down here. This is due to a special property of water: the elasticity of H2O. We know that when the temperature sinks below freezing, water first contracts and then expands as it begins to turn to ice. Ice, being lighter than water, floats.
Explanation:
The property of water that allows fish to survive in a lake that is frozen over is the fact that ice is less dense than liquid water.
How many grams of iron (III) oxide can be produced from 2.50 g of oxygen reacting with iron, according to the
following equation?
4 Fe (s) + 3 02 (g) -->2 Fe₂O3(s)
Answers
3 moles of oxygen gives 2 moles of the product. Then, 2.50 g or 0.07 moles of oxygen gas will give, 0.04 moles or 14.8 g of Fe₂O₃.
What is Fe₂O₃ ?Metals are easily reactive towards atmospheric oxygen and they form their oxides. Fe reacts with oxygen to form one of its oxide in the + 3 oxidation state that is Fe₂O₃.
Here, 3 moles of oxygen gives 2 moles of the oxide.
molar mass of oxygen gas = 32 g/mol
no.of moles in 2.5 g = 2.5 /32 = 0.07 moles.
0.07 moles produce, 0.07 × 2 /3 = 0.04 moles.
molar mass of Fe₂O₃ = 319.2 g.
then, mass of 0.04 moles = 0.04 × 319.2 = 14.8 g.
Therefore, 2.5 g of oxygen gas will give 14.8 g of the product.
Find more on Fe₂O₃:
https://brainly.com/question/24236942
#SPJ1
please help i will mark brainlist

Answers
Answer:
V= 21 cubic cm (( I am not sure about density ))
Explanation:
V= L×W×H
You shoot a beam of 4.5 eV light at a metal surface and notice that electrons are being
released from the metal. What will happen if you then increase the intensity of the 4.5
eV light?
O The metal will bend and warp.
O Nothing, the energy of the light is the same.
O More electrons would be released.
Photons would come off at higher speeds.
Answers
If you then increase the intensity of the 4.5 eV light, you will notice that the photons would come off at higher speeds.
What is intensity of light?
Light intensity refers to the strength or amount of light produced by a specific lamp source.
Intensity of light measures of the wavelength-weighted power emitted by a light source.
Mathematically, the intensity of light source is given as;
I = P/A
where;
P is the power of the incident light (photon energy per second )A is the unit areaIncreasing the light intensity (photon energy per second per unit area) increases the rate at which electrons leave the metal, and the electrons have more kinetic energy.
Thus, if you then increase the intensity of the 4.5 eV light, you will notice that the photons would come off at higher speeds.
Learn more about intensity of light here: https://brainly.com/question/28145811
#SPJ1
Are species A and B more closely related than species A and D? Answer yes or no and support your answer with information from the diagram.
Answers
Answer:
Please show diagram.
Explanation:
Thanks!
1) Photosynthesis can be represented by
6C02 (g) + 6 H20 (1) = C6H1206(s) + 602(g)
ЛН° = 2801 kJ
Explain how the equilibrium would be affected by the following changes:
a) partial pressure of CO2 is increased
b) 02 is removed from the mixture
c) part of the C6H1206(s) is removed from the mixture
d) more water is added
e) a catalyst is added
f) temperature is decreased.

Answers
The effect on equilibrium in the following cases: a)the equilibrium would shift towards the product side (right), b) shift towards the product side (right), c) shift towards the reactant side (left), d) shift towards the reactant side (left), e) it would not affect the position of the equilibrium, f) the equilibrium would shift towards the reactant side (left).
What is photosynthesis?Photosynthesis is the process by which plants, algae, and some bacteria convert light energy into chemical energy. During photosynthesis, light energy is absorbed by pigments called chlorophyll, which is located in the chloroplasts of plant cells. This energy is then used to power a series of chemical reactions that convert carbon dioxide and water into glucose (a type of sugar) and oxygen.
The overall equation for photosynthesis is:
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
In this equation, carbon dioxide (CO₂) and water (H₂O) are combined in the presence of light energy to produce glucose (C₆H₁₂O₆ ) and oxygen (O₂). The glucose produced by photosynthesis is used by the plant as a source of energy, while the oxygen is released into the atmosphere as a byproduct. Photosynthesis plays a vital role in the Earth's carbon cycle and is responsible for producing the oxygen that is essential for life on Earth.
Learn more about equilibrium here:
https://brainly.com/question/30807709
#SPJ1
• I feel confident about working gas problems that involve temperatures. because...
• In order to remember the ideal gas law, one strategy I used was....
• If I am not sure how to figure out the relationship between properties of an enclosed gas, one strategy I can use is...
Answers
The ideal gas law is an important equation that describes the behavior of gases, and for solving ideal gas problems.
How to remember ideal gas law?To help remember the ideal gas law, one strategy is to use the acronym PV = nRT
Where;
P is pressureV is volumen is the number of moles of gasR is the gas constantT is temperatureThis acronym can help you remember the variables involved in the equation and their relationships.
So if you're not sure how to figure out the relationship between properties of an enclosed gas, one strategy you can use is to apply the ideal gas law. You can rearrange the equation to solve for the variable you're interested in.
Learn more about ideal gas law here: https://brainly.com/question/12873752
#SPJ1
PLS HELP ASAP I WILL GIVE BRAINLIEST FOR THE ANSWER THAT IS RIGHT!Based on the diagram below, in what period is the atom located on the periodic table? *a. period 4b. period 2c. period 6d. period 1
Answers
Answer:
6
Explanation:
im guessing because there is no diagram
Please help me
Vote you brainiest but please just help

Answers
Explanation: The family traveled 80 km/hr for an hour and then trabeled 40 km/ hr for 2 hours. So they traveled 80 km in one hour then 80 km in the next 2 hours. Total they traveled 160 km in 3 hours.
Average speed = distance traveled/ taken time
= 160/3 = 53.33
what type of reaction takes place between methane and chlorine
Answers
Answer and Explanation:
When methane (CH4) and chlorine (Cl2) react, a substitution reaction takes place. Specifically, the reaction is a halogenation reaction, in which one or more hydrogen atoms in the methane molecule are replaced by chlorine atoms. The reaction can be represented by the following equation:
CH4 + Cl2 → CH3Cl + HCl
In this equation, CH3Cl represents chloromethane, which is a type of organochlorine compound. The reaction is typically initiated by ultraviolet (UV) light or heat, and it proceeds through a series of free radical chain reactions. The products of the reaction are typically a mixture of chloromethane and hydrogen chloride (HCl) gas.