Silver reacts with hydrogen sulphide gas, and oxygen according to the reaction: 4Ag(s) + 2H2S(g) + O2(g) → 2Ag2S(s)+ 2H2O(g) How many grams of silver sulphide are formed when 1. 90 g of silver reacts with 0. 280 g of hydrogen sulphide and 0. 160 g of oxygen?
Answers
When 90 g of silver reacts with 0.280 g of hydrogen sulphide and 0.160 g of oxygen, approximately 1.018 grams of silver sulphide are formed.
To determine how many grams of silver sulphide (Ag2S) are formed when 90 g of silver (Ag) reacts with 0.280 g of hydrogen sulphide (H2S) and 0.160 g of oxygen (O2), follow these steps:
1. Calculate the moles of each reactant using their molar masses:
- Silver (Ag): 90 g / (107.87 g/mol) ≈ 0.834 moles
- Hydrogen Sulphide (H2S): 0.280 g / (34.08 g/mol) ≈ 0.00821 moles
- Oxygen (O2): 0.160 g / (32.00 g/mol) ≈ 0.00500 moles
2. Determine the limiting reactant using the stoichiometry of the balanced chemical equation:
- Ag: 0.834 moles / 4 = 0.2085
- H2S: 0.00821 moles / 2 = 0.00411
- O2: 0.00500 moles / 1 = 0.00500
The smallest value is 0.00411, which corresponds to H2S, making it the limiting reactant.
3. Calculate the moles of Ag2S produced using the stoichiometry from the balanced chemical equation:
- Moles of Ag2S = 0.00411 moles H2S × (2 moles Ag2S / 2 moles H2S) = 0.00411 moles Ag2S
4. Convert the moles of Ag2S to grams using its molar mass (247.87 g/mol):
- Grams of Ag2S = 0.00411 moles Ag2S × 247.87 g/mol = 1.018 g Ag2S
So, when 90 g of silver reacts with 0.280 g of hydrogen sulphide and 0.160 g of oxygen, approximately 1.018 grams of silver sulphide are formed.
To know more about silver sulphide refer here: https://brainly.com/question/12753006#
#SPJ11
Related Questions
Which of the following is an example of the geosphere interacting with the atmosphere?Streams become muddy due to erosion.
Volcanic eruptions release gases into the air.
Rocks crack open when water in the cracks freeze.
Plants absorb carbon dioxide and produce oxygen.
Answers
Answer:
Volcanic eruptions release gases into the air.
Explanation:
This is because the atmosphere collects the gas from the volcano, the volcano is apart of the geosphere because it is a part of the earth's surfaces.
what does gravity cause causes
Answers
Answer:
position, speed and acceleration
How does temperature affect physical and chemical changes ?
Answers

What type of reaction is: ammonium nitrate -> dinitrogen monoxide + water?
Answers
Answer:
The thermal decomposition of ammonium nitrate to produce dinitrogen monoxide and water. This reaction takes place at a temperature of 200-260°C.
The arrangement of particles is most ordered in a sample of
1.
NaCl(aq)
2.
NaCl(l)
3.
NaCl(g)
4.
NaCl(s)
PLEASE HELP
Answers
We have that the arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
From the question we are told
The arrangement of particles is most ordered in a sample of
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
NaCl Generally known as sodium chloride or salt exist in four main states as shown
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
Mow in a subject of its arrangement of particles we can see that as its state changes from gaseous through to solid it gains in form and arrangement of particles
Therefore
The arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
For more information on this visit
https://brainly.com/question/1641336
if the pi for a particular amino acid is 7.5, at which ph will the net charge on the molecule be 1-?
Answers
The net charge on a molecule of a particular amino acid will be 1- when the pH is higher than the pI (isoelectric point) of the amino acid. In this case, the pI is 7.5, so the net charge will be 1- at a pH higher than 7.5.
The net charge on a molecule of an amino acid depends on the pH of the solution it is in. Amino acids have both acidic and basic functional groups, such as the amino group (-NH2) and the carboxyl group (-COOH). These functional groups can ionize, meaning they can either donate or accept protons depending on the pH of the solution.
The isoelectric point (pI) of an amino acid is the pH at which the net charge on the molecule is zero. At this pH, the number of positive charges (from protonated amino groups) is equal to the number of negative charges (from deprotonated carboxyl groups).
In this case, the pI of the amino acid is 7.5. Since the desired net charge is 1-, the pH must be higher than the pI. At a pH higher than 7.5, the amino acid will have a net negative charge because the carboxyl group will be deprotonated (COO-) while the amino group will remain protonated (NH3+). This results in a net charge of 1- on the molecule.
To learn more about isoelectric refer:
https://brainly.com/question/32069636
#SPJ11
Which one is which thank you

Answers
Objects feels warmer because more light is absorbed
Object appears white because all light is reflected
Object appears black because all light is absorbed
Object feels cooler because less light is absorbed
How reflection of light affect the color of the material seen?Reflection of light can affect the color of the material seen in several ways, depending on the properties of the material and the angle of incidence of the light.
When light strikes a surface, some of it is absorbed by the material, while the rest is reflected. The reflected light can be either specular, meaning it reflects at a single angle, or diffuse, meaning it reflects in multiple directions. The color of the material seen depends on the wavelengths of light that are reflected back to the observer.
Learn more about light:https://brainly.com/question/15200315
#SPJ1
the liquid dispensed from a burette is called ___________. select one: solute water titrant analyte
Answers
The liquid dispensed from a burette is called titrant. What is burette? A burette is a laboratory equipment used in analytical chemistry to dispense volumes of liquid precisely and accurately. A burette is used to deliver a variable, measured amount of liquid, and it is calibrated to enable a scientist to determine the volume of liquid it contains to an accurate level. What is titrant? Titrant is a liquid substance with a known concentration. Titrant is used in analytical chemistry to determine the concentration of an analyte (a chemical species under analysis). The amount of titrant required to react with a particular quantity of analyte is measured, and the concentration of the analyte is calculated from this titration.
#SPJ11
Learn more about titration:
https://brainly.com/question/186765
a sample of blood is found to contain 64.5 micrograms of valproic acid. how many milligrams of valproic acid does this blood sample contain?
Answers
The valproic acid contains 0.0645 milligrams
Conversion scale1000 microgram = 1 milligram
Data obatined from the questionFrom the question given, the following data were obtained:
Mass (in micrograms) = 64.5 microgramsMass (in milligrams) =?How to convert 64.5 micrograms to milligramsWe can convert 64.5 micrograms to milligrams as illustrated below:
1000 microgram = 1 milligram
Therefore,
64.5 micrograms = (64.5 micrograms × 1 milligram) / 1000 microgram
64.5 micrograms = 0.0645 milligrams
Thus, the valproic acid contains 0.0645 milligrams
Learn more about conversion:
https://brainly.com/question/1444594
#SPJ1
Please help it is very needed

Answers
Answer: probably c i'm not too good with chemistry however i can tell u how got it
Explanation: 27/13Al+1/0
or
simplify 27lA>13 which = 1>0
in my terms 1/0n the answer is c
btw no worries i tend to add a lot of people on brainly and when i send them a request i try to help them the best i can regardless of the trouble it takes, i'm not some crazy weirdo with a crazy crush, that's what all my friends thought, but nah not true i just like to help anyways.. uhm lemme stop typing
the minimum temperature at which a flame will ignite and continue to burn fuel oil vapor as it rises from a pool of liquid fuel oil is called the .
Answers
The minimum temperature at which a flame will ignite and continue to burn fuel oil vapor as it rises from a pool of liquid fuel oil is called the ignition point.
The ignition point can be defined as the minimum temperature at which the substance will continue to burn without any additional external heat of supply. the ignition point is also called as the kindling point. The Auto ignition point can be defined as the temperature of the substance at which the substance must reach before it ignite in the absence of the flame, but the air is present.
The lowest amount of the energy required for the ignition is called as the ignition energy.
To learn more about ignition here
https://brainly.com/question/6481027
#SPJ4
A neutral atom of beryllium (Be) has an average mass of 9 amu and 4 electrons. How many neutrons does it have?
Answers
Answer:
I think the answer could be 13 i hope this helps
hich option is an ionic compound?
Responses
NO2
upper case N O subscript 2 end subscript
SO3
upper case S O subscript 3 end subscript
CO
upper case C O
LiCl
Answers
NO₂ , SO₃ and CO are covalent compounds and LiCl is ionic in nature.
What are differences between covalent and ionic compounds?The definition of an ionic compound is chemical compound composed of ions which is held together by electrostatic forces i.e. held together by ionic bonds. They are formed by ions of opposite charge. The compound is neutral but it consists of a positively and negatively charged cations and anions.
Ionic bonds transfer electrons, covalent bonds share them more easily .Ionic compounds tend to have higher melting points and boiling points while covalent compounds have lower melting & boiling pointsIonic compounds have more polar molecules and covalent compounds lessOrganic compounds tend to have covalent bondsIonic compounds are usually between metal and a non-metal. Non-metal with non-metal compounds are covalent.Ionic compounds have ions in solution or in molten state and conduct electricityIonic bonds are stronger than covalent bondsIonic compounds tend to be a solid with definite shape at room temperature while covalent compounds are usually gases, liquids or soft solidsIonic compounds often do not dissolve in organic solvents while covalent compounds do.Learn more about ionic compounds at https://brainly.com/question/2687188
#SPJ10
Calculate the maximum wavelength of light capable of dissociating the i–i bond in one molecule of iodine if the bond energy, or bond dissociation energy, is 153 kj/mol.
Answers
The iodine molecule's i-i bond can be broken by light at a maximum wavelength of =782.39 nm.
The wavelength, what is it?A waveform signal's wavelength is defined as the separation between two identical points (adjacent crests) in adjacent cycles as the signal travels through space or along a wire. This length in wireless systems is typically expressed in meters (m), centimeters (cm), or millimeters (mm) (mm).
Light with a specific wavelength has the following energy:
E = hc/λ
E = energy of light
h = planck's constant = 6.626*10⁻³⁴J-s
c = speed of light = 3*10⁸ m/s
We are given bond energy of one mole i–i , but we are required to dissociate one molecule of bromine monochloride bond.
Bond energy of one mole i–i = 153kJ/mol ( 1 mol = 6.022*10²³ )
Bond energy of one molecule of i–i = 153/6.022*10²³ kJ/molecule (1kJ = 1000J)
E = (153)*(1000)/(6.022*10²³ )J/molecule (Multiplied 1000 to change kJ to J)
E = hc/λ
153*(1000)/6.022*10²³ = 6.626*10⁻³⁴*3*10⁸×λ
λ = 782.39nm
To know more about Wavelength visit:
https://brainly.com/question/1263539
#SPJ4
99.9% of the air particles are found in the lower 50 kilometres of the atmosphere. What does this say about the density of the air near Earth’s surface?
Answers
It means that the air near the Earth's surface is denser compared to the other parts.
Air Density99.9% of the air particles are found in the lower part of the atmosphere because the air in this region is denser than the other regions.
Air density increases with increased pressure and as we move up in altitude, air pressure decreases.
Thus, as air pressure decreases with height, the density of air also decreases. This is why most of the air particles are found in the lower part of the atmosphere.
More on air density can be found here: https://brainly.com/question/23294784?referrer=searchResults
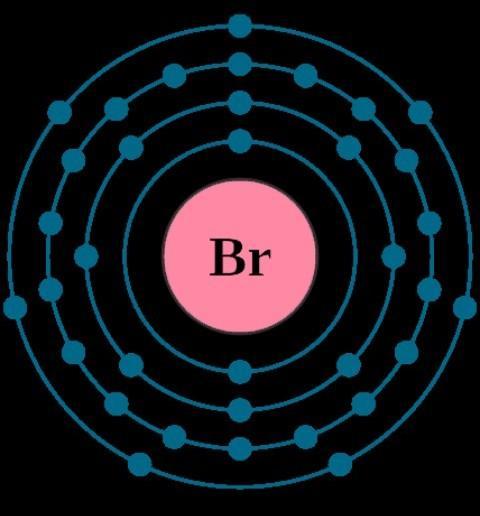
Which element will gain one electron in an ionic bond?
Select one:
a. Al
b. Cu
c. Br
d. O
Answers
Answer:
C. Bromine
Explanation:In ionic bond , the metals looses electron and the non metal gains electron.
In the given options ;
Aluminum (Al)= METAL
Copper (Cu) = METAL
Bromine (Br) = NON METAL
Oxygen (O) = NON METAL
That leaves us with bromine and oxygen.
Oxygen has an electronic configuration of 2,6.(see image).
So oxygen needs to gain two electrons.
Bromine has one valence electron ( see image).
So therefore , Bromine needs to gain one electron as It is in Group VII



Aspirin (C9H8O4) is synthesized by the reaction of salicylic acid (C7H6O3) with acetic
anhydride, C4H6O3. 2 C7H6O3 + C4H6O3 −→ 2 C9H8O4 + H2O. How much of the excess reactant is used when the reaction is complete? Answer in units of mol.
Answers
The amount of excess acetic anhydride is:Amount of excess acetic anhydride = initial amount - amount used = 0.0196 mol - 0.0145 mol = 0.0051 molTherefore, 0.0051 mol of acetic anhydride is used in the reaction.
The balanced chemical equation for the reaction of salicylic acid with acetic anhydride is given as follows: 2C7H6O3 + C4H6O3 ⟶ 2C9H8O4 + H2OIn this equation, salicylic acid (C7H6O3) is the limiting reagent and acetic anhydride (C4H6O3) is the excess reagent. The stoichiometric ratio between salicylic acid and acetic anhydride is 2:1. This means that for every two moles of salicylic acid, one mole of acetic anhydride is required. To find out how much of the excess reactant is used when the reaction is complete, we need to determine the limiting reagent and the excess reagent. We can do this by calculating the amount of product that each reactant can produce and comparing the values.Let's first calculate the number of moles of each reactant:No. of moles of salicylic acid = mass/molar mass = 2/138 = 0.0145 molNo. of moles of acetic anhydride = mass/molar mass = 2/102 = 0.0196 molTo determine the limiting reagent, we need to calculate the amount of product that each reactant can produce.
According to the balanced equation, 2 moles of salicylic acid produces 2 moles of aspirin, while 1 mole of acetic anhydride produces 2 moles of aspirin. Therefore, the amount of aspirin that can be produced from each reactant is as follows : Amount of aspirin produced from salicylic acid = 2 x 0.0145 mol = 0.0290 molAmount of aspirin produced from acetic anhydride = 2 x 0.0196 mol = 0.0392 molSince salicylic acid can produce only 0.0290 mol of aspirin, it is the limiting reagent. This means that acetic anhydride is in excess. To determine how much of the excess reactant is used, we need to subtract the amount of acetic anhydride used from the amount that was initially present. The amount of acetic anhydride used is equal to the amount of salicylic acid used, which is 0.0145 mol.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
EASYYY!!!!!
What arguments could you use to convince these farmers to start using some organic farming practises?
Answers
Answer:
- It's the right thing to do, duh
- In the long run, it will save their industry
- It might be cheaper
- It might become law soon, so they might as well get with it
I can't think of anything else, I hope it helps anyway!
which location on the map above marks the Weddell Sea?(Giving brainlist for correct answer)
-A
-B
-C
-D

Answers
Answer and Explanation:
D. I think..
When a weak base is titrated with a strong acid the equivalence point is?
Answers
When a weak base is titrated with a strong acid, the equivalence point is: a weak acid's formation.
The equivalence point happens when the moles of the acid equal the moles of the weak base, and pH = 7. It is because the hydronium ion concentration is identical to the hydroxide ion concentration in pure water at this pH. The pH of a weak base solution can be measured during titration.
The reaction between weak bases and strong acids happens in two steps. The first step is the formation of salt through the reaction between acid and base, and the second step is the hydrolysis of this salt. Anions derived from weak bases react with water and accept protons to produce hydroxide ions.
On the other hand, the cations derived from strong acids do not hydrolyze to produce acidic solutions. They can neither react with the acid nor the water, so the only effect is an increase in the concentration of cations in the solution.
The weak base's concentration before and after the equivalence point is much less than the weak acid concentration that is produced at the equivalence point. During the titration, the pH increases slowly initially, but it rises rapidly near the equivalence point, where the weak acid is produced.
After the equivalence point, the pH of the solution is lower because the excess of strong acid has changed the buffer solution into a weak acid solution.
To know more about weak acids refer here:
https://brainly.com/question/22104949#
#SPJ11
If the cell potential is negative, which electrode is connected to the anode?.
Answers
Answer: Heyo Kenji Here! Here's your answer- In addition, since the external battery source is what drives the electrons through the circuit, the electrodes will match the positive and negative terminal of the battery. While the anode remains the site of oxidation, it becomes the positive terminal, and the cathode becomes the negative terminal.
Explanation: Hope this helps!
Have a nice day!
- Kenji ^^
Answer: In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of electrons and cathode is where the electrons flow. However, in an electrolytic cell, the anode is taken to be positive while the cathode is now negative.
Explanation: I hope this helps!
What are the correct coefficients when this equation is balanced?
___ Sb + __ O2 --> Sb4O6
Answers
Answer:
4 Sb, 3 \(O_{2}\)
Explanation:
On the reactant's side of the equation (the left side), there is one Antimony and one Oxygen gas molecule (\(O_{2}\)). The oxygen gas molecule is made of two atoms, so we actually have 2 oxygens on the left side. On the product's side (the right side), there are 4 antimony atoms, and 6 oxygen atoms. If we were to write it out in a certain way, it would look like this:
__Sb + __ \(O_{2}\) --> \(Sb_{4} O_{6}\)
1 Sb 4
2 O 6
To balance this equation, those numbers on either side of the elements must equal each other. We can accomplish this with the proper coefficients. If we put a 4 in front of the antimony, it means this:
4 Sb + __ \(O_{2}\) --> \(Sb_{4} O_{6}\)
4 Sb 4
2 O 6
And the antimony is now balanced.
Now we must balance the oxygen. There are 6 oxygens on the product's side but only 2 on the reactant's side. To fix this, simply multiply the oxygen by 3:
4 Sb + 3 \(O_{2}\) --> \(Sb_{4} O_{6}\)
4 Sb 4
6 O 6
3 * 2 = 6, so now oxygen is balanced, and the equation is now correct.
Titration of valine by a strong base, for example NaOH, reveals two pK's. The titration reaction occurring at pK2 (pK2 = 9.62) is: A) —COOH + OH− → —COO− + H2O. B) —COOH + —NH2 → —COO− + —NH2+. C) —COO− + —NH2+ → —COOH + —NH2. D) —NH3+ + OH− → —NH2 + H2O. E) —NH2 + OH− → —NH− + H2O.
Answers
The correct answer is C) —COO− + —NH2+ → —COOH + —NH2. This is because pK2 refers to the second dissociation constant of the amino acid valine, which is the dissociation of the carboxyl group (—COOH) from the amino group (—NH2+).
How to find the titration reaction at pK\(_{b}\)?During titration with a strong base like NaOH, the base will react with the acidic proton (H+) on the carboxyl group, resulting in the formation of the carboxylate ion (—COO−) and water (H2O). However, once all the carboxyl groups have been neutralized, the excess base will react with the amino group (—NH2+) and remove the proton (H+) from it, resulting in the formation of the amino ion (—NH2) and water (H2O). This is the point at which pK2 is reached, and the reaction is represented by the equation C) —COO− + —NH2+ → —COOH + —NH2. The other answer choices are not relevant to the titration of valine with a strong base.
To know more about pK\(_{b}\) values:
https://brainly.com/question/31604314
#SPJ11
Edward is gathering physical evidence at the scene of a crime. He finds a fingerprint pressed into the wax of a candle. What type of fingerprint is this?
Answers
The type of fingerprint found at the crime scene in this scenario is referred to as plastic.
What is Plastic fingerprint?This is the of fingerprint which is three dimensional and can be easily seen with the human eye.
Plastic fingerprint can be made by pressing the finger on substances such as wax, paint etc.
Read more about Fingerprint here https://brainly.com/question/11165604
#SPJ1
Answer:
the answer would be molded I believe.
choices are Latent,patent, inadmissible molded,trace.
Photosynthesis Notes
1. Give the Greek root word meaning for photoautotroph.
2. What was von Helmont testing with his willow tree experiment? What was the result?
3. Define the law of conservation of mass.
4.
Describe the experiment used by Joseph Priestly to discover oxygen.
5. List the primary constituents of air.
a.
b.
C.
d.
e.
6. What did Jan Ingenhousz discover about plants?
7. Based on the work of Nicolas de Saussure, what are the two primary sources of mass for
plants?
8. Define photosynthesis-
9. Define potential energy-
Answers
Photoautotroph has been derived from the combination of three words, photo -meaning "light", autos- meaning "self", and troph meaning "nutrition".
Land plants and photosynthetic algae are both photoautotrophs. These species contain pigments that can capture light, like chlorophyll. Origin of the word: photo- (light) + auto (self) + troph (nourishment). Autotrophs known as photoautotrophs generate complex chemical substances like proteins, lipids, and carbohydrates by absorbing light. Photosynthesis is the name given to this light-mediated process.
An energy-rich carbohydrate like glucose is produced by plants using carbon dioxide, inorganic salts, and water during a process called photosynthesis. As a byproduct, oxygen is also produced, other than glucose. Land plants and photosynthetic algae are both photoautotrophs. These species contain pigments that can capture light, like chlorophyll.
To know more about autotrophs, refer to the following link:
https://brainly.com/question/28623341
#SPJ4
How do you find the molar mass of Cl?
Answers
The molar mass of Chlorine (Cl) can be calculated by adding up the atomic masses of all the atoms in one mole of the substance.
In the case of Chlorine, it has an atomic number of 17 and its atomic mass is 35.5 g/mol.
Therefore, the molar mass of Chlorine (Cl) is 35.5 g/mol. This means that one mole of Chlorine (Cl) weighs 35.5 grams. The molar mass is a fundamental property that helps in determining the chemical formula, calculating the number of particles in a given mass, and determining the amount of substance in a sample.
To know more about molar mass click here:
https://brainly.com/question/28296920#
#SPJ11
How do you stop a chemical burn from burning?
Answers
Remove dry chemicals.Remove contaminated clothing or jewelry. Bandage the burn. Rinse again if needed.
the amount of harm to the skin relies upon on how strong the chemical was, how lots of it become at the skin, and how long it become there. Chemical burns, even minor ones, can be very painful. A minor burn may heal inside a few days. however a extra extreme burn might also take weeks or maybe months to heal completely.you need to now not use ice, or even ice-bloodless water, on a burn. extreme cold carried out to a burn can further damage the tissue. to properly cool and clean a burn, remove any apparel that covers it.
To know more about chemicals click here
https://brainly.com/question/26487468
#SPJ4
What is the unit charge on each subatomic particles
Answers
Answer:
Protons, Electrons, Neutrons
Explanation:
Neutron: 0 or Neutral
Electron: -1 or Negative
Proton: +1 or Positive
For proton and neutron look at neutr for neutral and p for positive
Answer:
❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️
The answer is Protons, Electrons, Neutrons
There is three of them.....
Hope this will help you
❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️❤️
Explanation:
Which properties is/are characteristic(s) of gases?
Answers
One of the characteristics of gases is that gases are highly compressible. The correct option is C.
Gases possess several characteristic properties that distinguish them from other states of matter such as solids and liquids. The key properties of gases are as follows:
1. Expansion: Gases have the ability to expand and fill the entire available space of a container. They lack a definite shape or volume, and their particles are widely spaced.
2. Compressibility: Gases are highly compressible compared to solids and liquids. Under increased pressure, the volume of a gas decreases significantly, allowing it to be compressed into a smaller space.
3. Fluidity: Gases flow readily and can be easily poured or transferred from one container to another. They do not exhibit any resistance to shear forces and can easily mix with other gases.
4. Low Density: Gases have a low density compared to solids and liquids. The particles in a gas are far apart, resulting in a low mass per unit volume.
5. Diffusion and Effusion: Gaseous particles are in constant random motion and undergo diffusion, spreading out and mixing with other gases. They also exhibit effusion, which refers to the escape of gas molecules through tiny openings or pores.
6. High Kinetic Energy: Gas molecules possess high kinetic energy and move rapidly in all directions. The average speed of gas particles increases with higher temperatures.
7. Pressure: Gases exert pressure on the walls of their container due to the collisions between the gas particles and the container. The pressure of a gas is directly proportional to its temperature and the number of gas particles.
These properties collectively characterize gases and are a result of the weak forces of attraction between gas particles, allowing them to move freely and independently.
The correct option is C) Gases are highly compressible.
To know more about gases refer here:
https://brainly.com/question/9696086#
#SPJ11
A) Gases have a definite shape.
B) Gases are not affected by changes in pressure.
C) Gases are highly compressible.
D) Gases have a high density.
answers please!!!!!!

Answers
(a) HCL(aq) + NaOH(aq) ---------------> ___ + ____
The answer will be NaCl and H2O
(b) An equation to show the reaction between hydrogen ions and hydrogen ions and hydroxide ions is given below :
H+ + OH− ⇄ H2O
This reaction is known as neutralization reaction.
(c) Joey could tell us the reaction is over only when we obtained salt and water from the given chemical reaction (NaOH + HCl). This a basically a neutralization reaction ( the reaction generally finished at equilibrium)
(d) pH is 7.
This is because at the ending point the moles of HCl = the moles of NaOH so all that is present is H2O, Cl–, and Na+. So, the pH is 7.
To know more about Neutralization Reaction here :
https://brainly.com/question/20038776?referrer=searchResults
#SPJ1