Sodium sulfate is added to a solution mixture containing potassium nitrate and barium nitrate. After centrifugation, which aqueous ions are present in the supernatant
Answers
Sodium sulfate is added to a solution mixture containing potassium nitrate and barium nitrate. After centrifugation, potassium ions (K+) and nitrate ions are present in the supernatant.
Using the centrifugal force, centrifugation is a mechanical process that divides particles from a solution based on their size, shape, density, medium viscosity, and rotor speed. Less dense components of the mixture migrate in the direction of the centrifuge's axis while denser components move away from it. By increasing the test tube's effective gravitational force, chemists and biologists can ensure that the precipitate (pellet) sinks swiftly and completely. Supernatant or supernate refers to the liquid that is still present above the precipitate. Potassium ions (K+) and nitrate ions are present in the supernatant.
To know more about centrifugation, here:
https://brainly.com/question/13521993
#SPJ4
Related Questions
The energy of the motion of the particles in the matter
2 points
Heat
Potential Energy
Thermal Energy
Total Internal Energy
Kinetic energy
Answers
I hope this help
can I get mark branliest
How do reactants and products participate in a chemical reaction?
A. One reactant and one product react to form a new reactant and a new product.
B. The reactants are compounds that react to form new compounds (the products).
C. The products react with each other to form new compounds (the reactants).
D. A product is broken down in a chemical reaction to form reactants.
Answers
Answer:
B. The reactants are compounds that react to form new compounds (the products).
Explanation:
yessirski what he said is what im doing. Thanks, guys! #BrainlyPremium
how many neutrons are present in the nucleus of a phosphorus-32 (32p) atom (see the figure above)?
Answers
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. It has a mass number of 32, which means that the sum of the protons and neutrons in its nucleus is 32. Phosphorus has an atomic number of 15, which tells us that it has 15 protons in its nucleus.
To determine the number of neutrons in the nucleus of a phosphorus-32 atom, we subtract the atomic number from the mass number:
Number of neutrons = Mass number - Atomic number
Number of neutrons = 32 - 15
Number of neutrons = 17
Therefore, there are 17 neutrons present in the nucleus of a phosphorus-32 (32P) atom. This is important to know because the number of neutrons in an atom affects its stability and reactivity, as well as its isotopic properties. Phosphorus-32, for example, is used in a variety of biological and medical applications because of its ability to emit beta radiation, which can be used to study cellular processes and treat certain medical conditions.
TO KNOW MORE ABOUT Phosphorus-32 CLICK THIS LINK -
brainly.com/question/17009879
#SPJ11
1. Name each of the following binary compounds or ionic compounds with polyatomic ions.
a) LiBr
b)Cal2
Answers
B)Calcium Iodide
WILL GIVE BRANLIEST!!
Carbon tetrachloride (CCl4) is
ionic
covalent
Answers
Answer:
Covalent
Explanation:
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds
Answer:
Carbon tetrachloride (CCl4) is ✔ covalent.
Answers to assignment question:
What properties would you expect for CCl4? Select all that apply.
It is likely a gas or liquid at room temperature.
It is likely soluble in water.
It will likely light up a bulb in a conductivity apparatus.
It will likely have a high melting point.
Calcium oxide (CaO) is ✔ ionic.
What properties would you expect for CaO? Select all that apply.
It is likely a gas or liquid at room temperature.
It is likely soluble in water.
It will likely light up a bulb in a conductivity apparatus.
It will likely have a high melting point.
Explanation:
all correct on edge! :D
Which of the following could you add to increase the solubility of CaBr2? A. HCl. B. HBr. C. H2SO4 D. NaNO3. E. KOH.
Answers
The following could you add to increase the solubility of CaBr2 are NaNO3 and KOH. Option D and Option E are correct.
Solubility refers to the degree to which a substance is dissolved in a solvent to create a homogeneous solution. The solubility of a solute can be influenced by several variables, including temperature, pressure, and the chemical properties of the solute and solvent.
When an ionic compound, such as calcium bromide (CaBr2), is added to water, the solubility of the compound determines the degree to which it will dissolve.The solubility of calcium bromide (CaBr2) in water is determined by the type and amount of other substances that are present in the water. If you want to increase the solubility of calcium bromide, you can add a substance that is soluble in water and that can be ionized.
When the added substance dissolves in water, it ionizes and generates ions that can interact with the calcium and bromide ions in CaBr2 and increase the solubility. Sodium nitrate (NaNO3) and potassium hydroxide (KOH) are two substances that can increase the solubility of calcium bromide.
Therefore, the correct answer is D. NaNO3. and E. KOH.
Learn more about solubility -
brainly.com/question/23946616
#SPJ11
You have two solutions and want to find how two chemicals react. Solution A has 100 mL of 1.0 M particle A. Solution B has 100 mL of 1.0 M particle B. You combine the two solutions and find the new concentrations of A and B to both be 0.50 M.
Answers
Answer:
There was no reaction
Explanation:
1.Did a reaction occur? Explain your answer. If a reaction occurred, also provide a possible chemical equation describing the reaction.
When a reaction occurs, the initial moles of each species decreases to produce a determined product. If the initial moles of reactant = final moles there was no reaction:
Initial moles A:
0.100L * (1.0mol / L) = 0.100 moles A
Final moles A:
0.200L * (0.5mol / L) = 0.100 moles A
Initial moles B:
0.100L * (1.0mol / L) = 0.100 moles B
Final moles B:
0.200L * (0.5mol / L) = 0.100 moles B
As the moles in the beginning = Moles after the mixture
There was no reaction
During a process called photoact, ________ give up an electron as a part of the light-dependent reactions.
Answers
Answer:
Chloroplasts?
Explanation:
How many particles are in 23 grams of carbon tetrafloride
Answers
Answer:
5.399772727e22 atoms/particles or 5.39 x 10^22 atoms/particles
Explanation:
so to convert you’re going to convert grams to moles and moles to atoms/particles:
grams to moles:
23g CF4 x 1 mol
———— = 0.26136 mol
88g (molar mass of CF4)
moles to atoms/particles:
[2.066 • 10^23 is avogadro's number and is always used to convert to atoms/particles]
0.26136 mol x 2.066 • 10^23
———————
1 mol
this gives you 5.399772727e22 atoms/particles
so 5.399772727e22 atoms/particles or 5.39 x 10^22 atoms/particles for scientific notation
hope this helps :)
Find the percentage dissociation and hydrogen ion concentration of 0.2 mol/dm^3 of ethanoic acid if the equilibrium constant of acid is 1.85x10^-5 mol/dm^3
Answers
The percentage dissociation is 0.97% and the hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
To find the percentage dissociation and hydrogen ion concentrationwe need to use the equation:
Ka = [H^+][A^-] / [HA]
where Ka is the equilibrium constant, [H^+] is the hydrogen ion concentration, [A^-] is the concentration of the dissociated form of the acid (acetate ion), and [HA] is the concentration of the undissociated form of the acid (ethanoic acid).
We know the concentration of ethanoic acid, [HA] = 0.2 mol/dm^3, and the value of the equilibrium constant, Ka = 1.85 x 10^-5 mol/dm^3.
Using the equation:
Ka = [H^+][A^-] / [HA]
1.85 x 10^-5 = [H^+][A^-] / 0.2
[H^+][A^-] = 1.85 x 10^-5 * 0.2
[H^+][A^-] = 3.7 x 10^-6 mol^2/dm^6
Since the concentration of the acetate ion is equal to the concentration of the hydrogen ion, [A^-] = [H^+].
So,
[H^+]^2 = 3.7 x 10^-6 mol^2/dm^6
[H^+] = sqrt(3.7 x 10^-6) = 1.93 x 10^-3 mol/dm^3
The hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
To find the percentage dissociation, we divide the concentration of the acetate ion by the initial concentration of ethanoic acid and multiply by 100:
Percentage dissociation = ([A^-] / [HA]) * 100
Percentage dissociation = (1.93 x 10^-3 / 0.2) * 100 = 0.97%.
Therefore, The percentage dissociation is 0.97% and the hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
Learn more about hydrogen ion concentration here: brainly.com/question/29022526
#SPJ1
When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are:
carbon monoxide (g) + water (l) carbon dioxide (g) + hydrogen (g)
Answers
The smallest possible integer coefficients for the balanced equation are 1, 1, 1, 1.
The balanced molecular equation for the reaction:
carbon monoxide (g) + water (l) → carbon dioxide (g) + hydrogen (g)
can be obtained by following the steps of balancing the atoms in the equation.
First, we count the number of atoms of each element on both sides of the equation.
On the left side, we have:
1 carbon atom (C)
1 oxygen atom (O)
1 hydrogen atom (H)
On the right side, we have:
1 carbon atom (C)
3 oxygen atoms (O)
2 hydrogen atoms (H)
To balance the equation, we need to add coefficients to each molecule on the left side and right side of the equation to make the number of atoms of each element equal on both sides.
The balanced equation is:
CO(g) + H₂O(l) → CO₂(g) + H₂(g)
Therefore, the coefficients for the balanced equation are:
CO: 1
H₂O: 1
CO₂: 1
H₂: 1
learn more about carbon here:
https://brainly.com/question/22530423
#SPJ11
You have been collecting data on a set of unidentified elements. To keep track of data each sample has been randomly assigned to a letter A-F. Below is the data that has been collected. Use it to answer the questions below. It has been determined that the unknown elements come from two different families. Use your data to sort the elements into two families into the table below. Be sure to explain what data you used and why you sorted them that way
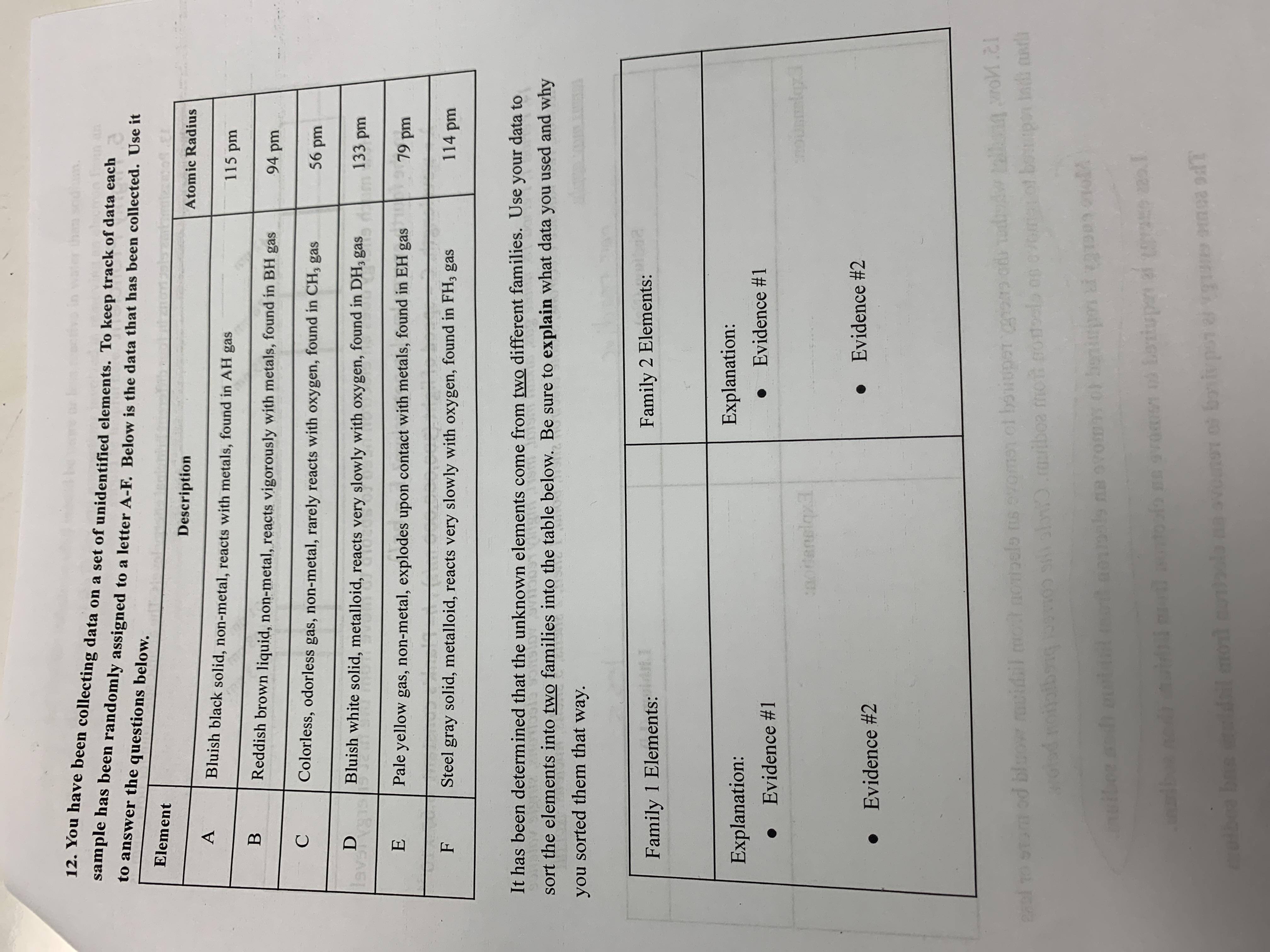
Answers
What are the families of the elements?
We know that the elements can be classified into families and the families into which the elements can be classified would depend on the reactivity of the elements and the number of electrons in the valence shell of the atoms of the elements.
We can see that the elements that are in family 1, A, E,B all form the kind of hydrides that have the formula AH. This implies that they are univalent and the there are non metallic.
Family 2 would comprise of the elements C, D F. these are the elements that form the compounds CH3 hence they must be trivalent nonmetals.
Learn more about families of elements:https://brainly.com/question/15846012
#SPJ1
In a solar cell, semiconductors of the p-type and n-type are placed in contact with each other via a conducting wire. In order to generate an electric current, which of the following must be true? A. Light shining on the system must have enough energy to set electrons in motion from the p-type to the n-type semiconductor. B. An external battery must be attached. C. Light shining on the system must have enough energy to set electrons in motion from the n-type to the p-type semiconductor. D. Light shining on the system will cause oxidation to occur.
Answers
The right response is A. To move electrons from the p-type semiconductor to the n-type semiconductor, light beaming on the system must have sufficient energy.
What is semiconductor?Semiconductors are substances that exhibit conductivity intermediate between that of conductors (often metals) and that of insulators or non-conductors (such as ceramics). Semiconductors can be pure elements like germanium or silicon or compounds like gallium arsenide.
The correct answer is A. Light shining on the system must have enough energy to set electrons in motion from the p-type to the n-type semiconductor.
When light shines on the solar cell, it excites electrons in the p-type semiconductor, allowing them to move across the interface to the n-type semiconductor. This creates a flow of electrons, which can be harnessed to generate an electric current. This process is known as the photovoltaic effect.
Option B is incorrect because an external battery is not required to generate an electric current in a solar cell. Option C is also incorrect because the electrons move from the p-type to the n-type semiconductor, not the other way around. Option D is also incorrect because oxidation does not play a role in the functioning of a solar cell.
Learn more about semiconductors on:
https://brainly.com/question/18132856
#SPJ11
in need of correct answer por favor

Answers
Answer:
B
Explanation:
because HCl donates a proton to water. Therefore, HCl is an acid. H2O accepts a proton from HCl. Therefore, H2O is a base.
Explain how patterns in the periodic table can provide evidence that explains how the structure of each element is different from another.
Answers
The elements are arranged in periods and groups. They are also arranged by the way they react to other chemicals, or the chemicals that are mixed in the element to make it.
The evidence that explains how the structure of each element is different from another is that the elements are arranged in periods and groups.
The periodic table refers to the tabular display of the chemical elements. It should be noted that the periodic table is arranged based on the increasing atomic number of the elements.
The elements that are in the periodic table are also arranged by the way they react to other chemicals. Therefore, the elements that are in a particular group typically have the same characteristics.
Read related link on:
https://brainly.com/question/5056924
What is the highest energy sub-shell occupied by electrons in a
titanium (Z=22) atom with a net electric charge of +2. Use a sketch
of the electronic configuration in your answer.
Answers
The highest energy subshell occupied by a Titanium ion with +2 charge (Ti⁺²) will be 4s.
The element Titanium has an Atomic Number of 22. This means that Titanium has 22 electrons bound by the nucleus, which are assigned to various orbitals. The order of the filling of the orbitals, which is the same for all elements, goes as follows for Titanium.
Ti₂₂ = 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d²
As per the order, the orbitals are written in the order of increasing energy, which can be checked by the (n + l) rule.
In the question, Ti⁺² ion is mentioned, where two electrons have been removed. Since the electrons are always removed from the outermost orbital, the electronic configuration of the ion will be:
Ti⁺² = 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁰
As seen, the electrons are removed from the outermost orbital. Thus, after removal, the highest energy orbital would be 4s.
(Image depicting Electronic Configuration for reference)
For more on Electronic Configuration,
brainly.com/question/30161188
#SPJ4

10. Water has a specific heat capacity that is about 10 times that of iron. In an experiment, a 50-g pellet of iron at a temperature of 200°C is dropped into 50 g of water at a temperature of 20°C.
When the system reaches thermal equilibrium, the temperature will be closest to
110°C
o'c
20°C
200°C
Answers
When the system reaches thermal equilibrium, the temperature will be closest to 20°C.
To determine the final temperature when the iron pellet is dropped into water, we can use the principle of heat transfer. The heat lost by the iron pellet will be equal to the heat gained by the water.
The heat lost by the iron pellet can be calculated using the equation:
Q_iron = m_iron * c_iron * ΔT_iron
where m_iron is the mass of the iron pellet, c_iron is the specific heat capacity of iron, and ΔT_iron is the change in temperature of the iron pellet.
The heat gained by the water can be calculated using the equation:
Q_water = m_water * c_water * ΔT_water
where m_water is the mass of the water, c_water is the specific heat capacity of water, and ΔT_water is the change in temperature of the water.
Since the total heat lost by the iron pellet is equal to the total heat gained by the water, we can set up the equation:
Q_iron = Q_water
m_iron * c_iron * ΔT_iron = m_water * c_water * ΔT_water
Substituting the given values:
(50 g) * (specific heat capacity of iron) * (final temperature - 200°C) = (50 g) * (specific heat capacity of water) * (final temperature - 20°C)
Since the specific heat capacity of water is approximately 10 times that of iron, we can assume that the specific heat capacity of iron is negligible compared to that of water. Therefore, we can approximate the equation as:
50 * (final temperature - 200) ≈ 500 * (final temperature - 20)
Simplifying the equation:
final temperature - 200 ≈ 10 * (final temperature - 20)
final temperature - 200 ≈ 10 * final temperature - 200
200 - 20 ≈ 10 * final temperature - final temperature
180 ≈ 9 * final temperature
final temperature ≈ 180 / 9
final temperature ≈ 20°C
Learn more about specific heat capacity, here:
https://brainly.com/question/1105305
#SPJ1
Any 1 help with Chemistry here??
Answers
5) Match the term with the correct definition.
Thermal Energy
O the science dealing with the conversion of heat and enery
O a nuclear reaction in which a nucleus of atoms is divided
O a
a nuclear reaction in which the nucleus of atoms are join
O the basic unit of measure for light
O the energy of heat
Answers
How many do each element are present in the following formula? 3 Ca3 (PO4)2 + 4 CaO
I really need help plz help if you can!!!!!
Answers
3 Ca3 (PO4)2 + 4 CaO
So we can see that there are 3 Ca3 (Ca3, Ca3, Ca3), which means you multiply the subscript by the coefficient, meaning 9 Ca. Then we see (PO4)2, which means that there are two PO4 (PO4, PO4) so we multiply the subscript 2 by the subscript 4 on O to equal 8 O, and we multiply the subscript 2 by the subscript 1 on P (the 1 is not written) to equal 2 P.
So far we have 9 Ca, 8 O, and 2 P.
One last term, 4 CaO (means CaO, CaO, CaO, CaO) which translates to 4 Ca and 4 O.
Add them all together and you get (9+4=13) 13 Ca, (8+4=12) 12 O, and 2 P
10pts! Will give brainllest if you are right! Please come quicklyyyy!
How are speed and velocity related?
A. they're exactly the same
B. velocity = speed and direction
C. speed = velocity and direction
D. direction = speed and velocity
Answers
Answer:
Hey there :))
Speed and Velocity are related in a lot of ways distance and displacement are. Speed is a scalar and velocity is a vector. They both represent a way to measure the change in position of an object relative to time.
Consider the intermediate equations:
3 equations. 1: upper C solid plus upper o subscript 2 gas right arrow upper C upper O subscript 2 gas Delta H 1 equals negative 393.5 kilojoules. 2: 2 upper C upper O gas plus upper O subscript 2 gas right arrow 2 upper C upper O subscript 2 gas Delta H 2 equals negative 566.0 kilojoules. 3: 2 upper H subscript 2 upper o gas right arrow 2 upper H subscript 2 gas plus upper O subscript 2 gas delta H 3 equals 483.6 kilojoules.
With the overall reaction:
Upper C (s) plus upper H subscript 2 upper O (g) right arrow upper C upper O (g) plus upper H subscript 2 (g).
What must be done to calculate the enthalpy of the reaction? Check all that apply.
The first equation must be halved.
The first equation must be reversed.
The second equation must be halved.
The second equation must be reversed.
The third equation must be halved.
The third equation must be reversed.
What is the overall enthalpy of the reaction?
Delta.Hrxn =
Answers
The overall enthalpy of the reaction is -131.3 kJ.
To calculate the overall enthalpy of the reaction, you need to manipulate the given equations and combine them in a way that cancels out the common substances. Let's analyze each equation:
1: C (s) + O₂ (g) -> CO₂ (g) ΔH₁ = -393.5 kJ
2: 2CO (g) + O₂ (g) -> 2CO₂ (g) ΔH₂ = -566.0 kJ
3: 2H₂O (g) -> 2H₂ (g) + O₂ (g) ΔH₃ = 483.6 kJ
To obtain the overall reaction: C (s) + H₂O (g) -> CO (g) + H₂(g), we can manipulate the equations as follows:
1: Reverse equation 1 to obtain: CO₂ (g) -> C (s) + O₂ (g) ΔH₁' = +393.5 kJ
2: Halve equation 2 to obtain: CO (g) + 1/2 O₂ (g) -> CO₂ (g) ΔH₂' = -283.0 kJ
3: Reverse and halve equation 3 to obtain: H₂ (g) + 1/2 O₂ (g) -> H₂O (g) ΔH₃' = -241.8 kJ
Now, we can sum up the manipulated equations to obtain the overall reaction:
CO₂ (g) + CO (g) + H₂ (g) + 1/2 O₂ (g) -> C (s) + 2CO₂ (g) + H₂O (g) + 1/2 O₂ (g)
To calculate the overall enthalpy change (ΔHrxn), we sum up the enthalpy changes of the manipulated equations:
ΔHrxn = ΔH₁' + ΔH₂' + ΔH₃'
= 393.5 kJ + (-283.0 kJ) + (-241.8 kJ)
= -131.3 kJ
Therefore, the overall enthalpy of the reaction is -131.3 kJ.
for more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
Make a table comparing atmospheric pressure versus ambient pressure:
Answers
Atmospheric pressure is the weight of the atmosphere above a certain point on the Earth's surface, whereas ambient pressure is the pressure of the surroundings. A comparison table between atmospheric pressure and ambient pressure can be drawn as follows:
Characteristics
Atmospheric Pressure
Ambient Pressure
Definition
The weight of the atmosphere above a certain point on the Earth's surface
The pressure of the surroundings
Measurement
In kPa or atm unitsIn kPa or atm units
Altitude
Above sea level
Not related to altitude
Units of measurement
In kPa or atm unitsIn kPa or atm units
EquationP = ρghP = F/A = Force/Area
Examples
Normal atmospheric pressure is 101.325 kPa
Normal ambient pressure is 101.325 kPa
Applications
Meteorology, weather forecasting, aviation, space exploration, etc.
Space exploration, pressurized chambers, HVAC systems, etc.
In summary, atmospheric pressure is the weight of the air molecules and ambient pressure is the pressure of the surroundings. These are both measured in kPa or atm units and are used in various fields such as meteorology, aviation, and space exploration.
To know more about Atmospheric pressure, visit;
https://brainly.com/question/31634228
#SPJ11
PLEASE HELP!!
George is writing an essay about the role of observation and inference in the development of the atomic theory. He wants to explain why it was more difficult to observe the presence of neutrons in atoms. Which statements should he include in his essay? Choose the two statements that apply.
A. While protons or electrons can be influenced by other charged particles, neutrons are not.
B. It was difficult to observe that neutrons were different than protons because the two particles respond to charge in the same way.
C. It was difficult to observe that neutrons were different than electrons because the two particles respond to charge in the same way.
D. Neutrons are held tightly together with protons in the nucleus, so scientists could not observe the behavior of neutrons independently.
Answers
Answer:
D and A
Explanation:
While protons or electrons can be influenced by other charged particles, neutrons are not.
and
Neutrons are held tightly together with protons in the nucleus, so scientists could not observe the behavior of neutrons independently.
Microgravity is a condition where an object or person can appear to be weightless. true or false
Answers
Answer:
its true
Explanation:
Cause i know because i just have notes a lot and i am in 10th grade U w U
How does a scientist make two solutions with the same molarity?
O A. By dissolving the same number of moles of each substance in the
same volume of water
B. By dissolving the same number of grams of each substance in the
same volume of water
C. By dissolving the maximum amount of each substance in the
same volume of water
D. By dissolving 1 mole of each substance in enough water to make
sure dissolving is complete
Answers
Answer:
a
Explanation:
It's a duh
Calculate the pressure, in atm, of 0. 0158 mole of methane (ch4) in a 0. 275 l flask at 27 °c
Answers
The pressure of 0.0158 mole of methane in a 0.275 L flask at 27 °C is approximately 4.42 atm.
To calculate the pressure of the methane in the flask, we can use the ideal gas law equation:
PV = nRT
Where:
P = Pressure (in atm)
V = Volume (in liters)
n = Number of moles
R = Ideal gas constant (0.0821 L·atm/(mol·K))
T = Temperature (in Kelvin)
First, let's convert the temperature from Celsius to Kelvin:
T(K) = T(°C) + 273.15
T(K) = 27 + 273.15
T(K) = 300.15 K
Now we can substitute the given values into the ideal gas law equation:
P * 0.275 = 0.0158 * 0.0821 * 300.15
Solving for P:
P = (0.0158 * 0.0821 * 300.15) / 0.275
P ≈ 4.42 atm
Therefore, the pressure of 0.0158 mole of methane in a 0.275 L flask at 27 °C is approximately 4.42 atm.
Learn more about pressure here
https://brainly.com/question/30673967
#SPJ11
I don’t understand what I am supposed to do with this

Answers
What variable represents specific heat in the equation Q = mcAT?
O A. The variable c
OB. The variable T
O C. The variable m
O D. The variable Q

Answers
Answer: A. The variable c
============================================
Explanation:
Q = heat transferred
m = mass
c = specific heat
\(\Delta T\) = delta T = change in temperature
Which correctly describe a reversible reaction reaching equilibrium in a closed system?
Select two that apply.
-Over time, the rates of the forward and reverse reactions equalize.
-Over time, the rate of the forward reaction becomes zero.
-Initially, the concentration of reactants is low, so the rate of the forward reaction is also low.
-Initially, the concentration of products is low, so the rate of the reverse reaction is also low.
-Over time, the rate of the reverse reaction becomes greater than the forward reaction.
Answers
Answer:
-Over time, the rates of the forward and reverse reactions equalize.
-Initially, the concentration of products is low, so the rate of the reverse reaction is also low.
Explanation:
A chemical reaction is said to be reversible when the reactants forms the products, which in turn reacts together again to give rise to the reactants. In a reversible reaction, the formation of products from reactants occurs simultaneously with the reformation of the reactants from the products. For example:
The reversible reaction: A + B ⇆ C + D means;
A + B → C + D and C + D → A + B
The rate at which both forward and reverse reactions are taking place in closed system may be initially different but with time, it gets equal to form an equilibrium reaction. However, at first, only the rate of the forward reaction proceeds because the concentration of the product is low. Hence, the rate of reaction of the reverse reaction (product to reactants) is low as well.
In the reversible reaction above, the rate of the reverse reaction (C + D → A + B) will turn out low initially because the concentration of the products (C and D) are low. With time, the rates of the forward and reverse reaction becomes equal to form an EQUILIBRIUM or STABLE reaction.