some compounds can be seen under uv light becasue they are inherently fluorescent and usually glow brightly
Answers
Some compounds can be seen under uv light becasue they are inherently fluorescent and usually glow brightly.
What are fluorescent compounds?
the light that a material emits when exposed to photons of a different wavelength. When exposed to UV radiation, a fluorescent object frequently emits visible light. The fluorescent properties of some deep sea fish and fireflies are examples of fluorescence in nature.
When a substance passes through fluorescence, it emits light after having absorbed light or other electromagnetic radiation. B2 emits a yellow colour while fluorescing. Tonic water glows blue because it contains quinine. Benzimidazoles, benzothiazoles, coumarins, vitamins like cyanocobalamine, fluorene, and pentacene are examples of fluorescent materials.
To learn more about fluorescent compounds from the given link below,
https://brainly.com/question/8979272
#SPJ4
Related Questions
the property of water molecules that is responsible for all the other physical and chemical properties is
Answers
The hydrogen bonding of water is one of the main property of water which makes it responsible for all the other physical and chemical properties.
Water molecules are polar in nature and they form hydrogen bonds. Hence, they have a high boiling point , high specific heat and density. Water can exhibit the properties of an acid, as well as a base (amphoteric character). The water molecules are constantly moving and the hydrogen bonds continuously breaks and forms again. These hydrogen bonds are strong, which is the reason for the unique properties of water.
To learn more about properties of water :
https://brainly.com/question/15395979
#SPJ4
How would you separate a mixture of the following compounds by extraction? Meo OH CHCHOH Select one: a. Dissolve both in DCM and extract with NaOH(aq): b. Dissolve both in DCM and extract with NaHCOz(aq) These cannot be separated by extraction as they have the same reactivity: d. Dissolve both in DCM and extract with HCI (aq)_
Answers
The best method for extracting the mixture's components would be choice b.
To separate a mixture of MeOH, CH3CHO, and CH3CHOH by extraction, we can use the difference in their acid-base properties. MeOH and CH3CHO are neutral molecules, while CH3CHOH can act as a weak acid and donate a proton to a base. Therefore, we can selectively extract CH3CHOH using a basic aqueous solution.
Option a suggests using NaOH(aq) to extract the mixture, which would convert CH3CHOH to its conjugate base CH3CHO^- and make it more water-soluble. However, this may also deprotonate MeOH and make it more water-soluble, reducing the efficiency of the extraction.
Option b suggests using NaHCO3(aq) as the extracting solution, which would selectively extract CH3CHOH without affecting MeOH or CH3CHO. The resulting CH3CHOH-NaHCO3 salt can be converted back to CH3CHOH by adding an acidic solution.
Option c suggests using HCl(aq) to extract the mixture, which would protonate CH3CHOH to form CH3CHOH2+ and make it more water-soluble. This may also protonate MeOH and CH3CHO, reducing the efficiency of the extraction.
Overall, option b would be the most effective way to separate the mixture by extraction.
To learn more about extraction refer to:
brainly.com/question/13646882
#SPJ4
chemistry question help please

Answers
Answer:
xii. B. Smelting
xiii. D. Carbon
Explanation:
Smelting is known to be a process by which a metal is gotten. It is gotten either as the element or as a simple compound. This is obtained from metal's ore by heating. The heating can be done ordinarily in the presence of oxidizing agents, like air, or reducing agents, carbon e.g coke.
Zinc is gotten after Zinc oxide is heated in the presence of carbon.
This process is smelting.
Concentrated tetraoxosulphate acid is a strong dehydrating agent. It has the ability to char wood, paper, or sugar, leaving a carbonaceous residue. The charring effect is as a result of the carbon that is found in the wood.
The acid sucks in the oxygen and hydrogen, leaving behind the carbon; this results in the charring effect.
What is the name of the the multi valence compound SnH4?
Answers
~ Hope this helps!
A 1,600-milliliter bucket of paint is at a temperature of 70°F. Then the paint is divided equally into four smaller buckets. Each bucket is at 70°F before the paint is poured.
the temperature of each 400 milliliter bucket is about _____ the temperature of the original bucket of paint.
a.the same as
b.four times
c.one fourth
science
Answers
Answer:
a.the same as
Explanation:
The temperature of each 400mL bucket is about the same as the temperature of the original bucket of paint.
This is because the temperature of the paint inside the bucket is not dependent on the amount of paint present.
In this regard, the temperature is an intensive property. So each of the boxes will have a temperature that is the same as that of the original bucket. Each of the particles have the same amount of kinetic energy.Identify an element as a metal or a non-metal according to its position in the Periodic
Table
Answers
Answer:
Metals on the left of the Periodic Table.
Non-Metals on the top-right, plus Hydrogen.
Would a tighter or a looser cover heat the oven faster? How about no covering at all?
Answers
What will the pressure be if 89.9 moles of argon are contained in a 12.0 L cylinder that is pressurized at a temperature of 300 K?
Answers
Done!
Zinc metal reacts with hydrochloric acid to produce zinc chloride and hydrogen gas as seen in the chemical equations below:
Zn(s) + 2HCl(aq) === ZnCl2(aq) + H2(g)
What type of equation is described above?
F. Double Replacement
G. Single Replacement
H. Decomposition
J. Synthesis
Answers
Answer:
Single Replacement.
Explanation:
what type of compound is sugar
Answers
Answer:
sucrose?
Explanation:
Answer:
sugar is an ionic compound
Explanation:
No links no bots please
which element is most likely to react with Br
Sr
Ar
K
O
Answers
There are two types of chemical compound one is covalent compound and another is ionic compound in chemistry. In ionic bonds, electrons are completely transferred. The correct option is option C.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
Covalent compounds are formed by covalent bond and ionic compounds are formed by ionic bond. Covalent bond is formed by sharing of electron and ionic bond are formed by complete transfer of electron. Ionic bonds are stronger than covalent bonds. The melting and boiling points are higher in ionic compounds.
Ionic bonds are formed by elements whose electronegativity difference is very large. Since K has high electronegativity among all given elements so K is most likely to react with Br.
Therefore, the correct option is option C.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
HELP ASAP
Two isotopes of the FAKE element Sz have the following abundances;
Seabreezium-71 75%
Seabreezium - 76 25%
What is the average atomic mass?
(Limit your answer to the TENTHS place.)
Answers
Two isotopes of the FAKE element Sz, Average mass of Seabreezium is equal to 199.77625 amu.
Given that Two isotopes of of Sz is given as :
Seabreezium-71 75%
Seabreezium - 76 25%
Now,
Isotopes of Seabreezium is given as:
Seabreezium-271Seabreezium - 269Now,
1st isotope = 71 75% of 271
= 194.44amu
2nd isotope = 76 25% of 269
= 205.11 amu
Now,
Average Mass of Seabreezium = 205.11 + 194.44/2
Average Mass of Seabreezium = 199.77625 amu
Thus, from the above conclusion we can say that, Average mass of Seabreezium is equal to 199.77625 amu.
Learn more about Average Mass here :https://brainly.com/question/24186882
#SPJ13
A ballon is filled with 0.328 moles of gas that has a volume of 7.28 L. If 0.135 moles are added to the ballon, how much would the new volume be?
Answers
If 0.135 moles are added to the balloon, its new volume would be 2.997 L.
Calculation-The optimal petrol law can be used to resolve this issue:
PV = nRT
where R is the gas constant, n is the number of moles, P is the pressure, V is the volume, and T is the temperature.
We may link the original and final volumes using the equation below, assuming constants for pressure and temperature:
(V1 / n1) = (V2 / n2)
If V1 is the starting volume, n1 is the starting mole count, V2 is the ending volume, and n2 is the ending mole count.
We may first determine the initial molar volume of the gas using the provided values:
V1/n1=7.28 L/0.38 mol = 22.195 L/mol
The final volume can then be determined using the molar volume:
0.135 mol x 22.195 L/mol = 2.997 L is what V2 = n2 x (V1 / n1) equals.
to know more about volume here:
brainly.com/question/1578538
#SPJ1
a researcher could say with certainty that someone was affected by the peripheral route to persuasion by conducting
Answers
A researcher could say with certainty that someone was affected by the peripheral route to persuasion by conducting a thorough analysis of the individual's behavior, attitude, and response to external cues such as social norms, authority, and attractiveness.
This would involve gathering data from various sources such as surveys, interviews, and experiments, and evaluating them against established theories and models of persuasion.A researcher could say with certainty that someone was affected by the peripheral route to persuasion by conducting a thorough analysis of the individual's behavior, attitude, and response to external cues such as social norms, authority, and attractiveness. By doing so, the researcher would be able to determine whether the individual was influenced primarily by peripheral cues such as superficial features or context, rather than by the central message or arguments presented.
Learn more about persuasion here
https://brainly.com/question/15157593
#SPJ11
why does liquid exerts pressure
Answers
Answer:
The particles of fluids are constantly moving in all directions at random. As the particles move, they keep bumping into each other and into anything else in their path. These collisions cause pressure, and the pressure is exerted equally in all directions.
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
1. Given the unbalanced equation:
C12H22O11 = C + H2O
If you produced 34.55 moles of C, how many moles of water were produced as well?
2. Given the unbalanced equation:
Cr + H2SO4 = CrSO4 + H2
If you want to make 14.81 moles of CrSO4, how many moles of Cr do you need?
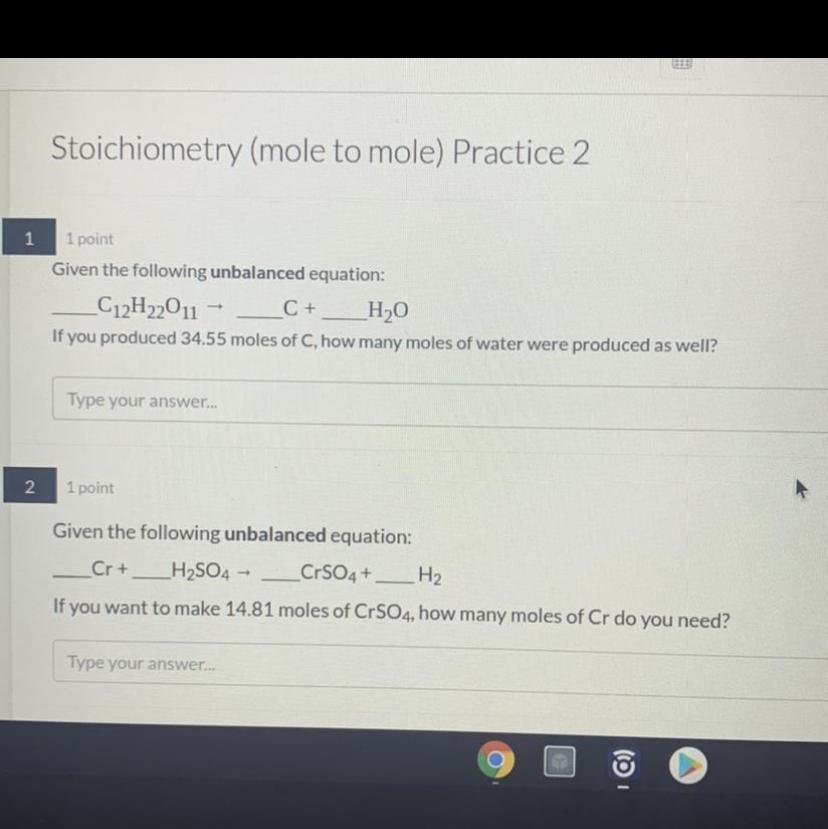
Answers
Answer:
1. 31.68 moles of water, H₂O
2. 14.81 moles of Cr
Explanation:
1. Determination of the number of mole of water, H₂O.
The balanced equation for the reaction is given below:
C₁₂H₂₂O₁₁ —> 12C + 11H₂O
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 12 moles of C and 11 moles of H₂O.
Next, we shall determine the number of mole C₁₂H₂₂O₁₁ needed to produce 34.55 moles of C. This can be obtained as follow:
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 12 moles of C.
Therefore, Xmol of C₁₂H₂₂O₁₁ will produce 34.55 moles of C i.e
Xmol of C₁₂H₂₂O₁₁ = 34.55 / 12
Xmol of C₁₂H₂₂O₁₁ = 2.88 moles
Thus, 2.88 moles of C₁₂H₂₂O₁₁ is needed.
Finally, we shall determine the number of mole of water, H₂O produced from the reaction. This can be obtained as follow:
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 11 moles of H₂O.
Therefore, 2.88 moles of C₁₂H₂₂O₁₁ will produce = 2.88 × 11 = 31.68 moles of H₂O.
Thus, 31.68 moles of water, H₂O were obtained from the reaction.
2. Determination of the number of mole of Cr needed.
The balanced equation for the reaction is given below:
Cr + H₂SO₄ —> CrSO₄ + H₂
From the balanced equation above,
1 mole of Cr reacted to produce 1 mole of CrSO₄.
Finally, we shall determine the number of mole of Cr needed to produce 14.81 moles of CrSO₄. This can be obtained as follow:
From the balanced equation above,
1 mole of Cr reacted to produce 1 mole of CrSO₄.
Therefore, 14.81 moles of Cr will also react to produce 14.81 moles of CrSO₄.
Thus, 14.81 moles of Cr is needed for the reaction.
how many ionic bonds are in copper?
Answers
The number of ionic bond in copper is zero.
Pure copper or any pure metal for that matter are examples of metallic bonds, which are neither ionic nor covalent. The copper atoms are stacked very tightly in a solid lattice. In fact it has a face centered cubic lattice which is the closed packing of atoms possible. Each atom has twelve nearest neighbors which allows their 4s orbitals (mostly) to have the optimal overlap. All of these orbitals therefore combine in one gigantic band of delocalized orbitals that spans the entire crystal with its myriad atoms. This band is only partly filled and the difference in energy between one state and the next is puny, which explains copper’s outstanding conductive properties.
Therefore, the bonding in copper has a metallic character.
To know more about copper bonding,
https://brainly.com/question/20897148
https://brainly.com/question/17215770
A chemist obtains a sample of
sodium sulfate, Na2SO4, with a mass of 71.0 g. How many formula units of sodium sulfate are in the sample?
Answers
The number of formula units of Na₂SO₄ present in the sample is 3.01×10²³ units
Avogadro's hypothesisFrom Avogadro's hypothesis,
1 mole of Na₂SO₄ = 6.02×10²³ units
But
1 mole of Na₂SO₄ = (23×2) + 32 + (16×4) = 142 g
Thus,
142 g of Na₂SO₄ = 6.02×10²³ units
How to determine the units in 71 g of sample142 g of Na₂SO₄ = 6.02×10²³ units
Therefore,
71 g of Na₂SO₄ = (71 × 6.02×10²³) / 142
71 g of Na₂SO₄ = 3.01×10²³ units
Thus, 3.01×10²³ units of Na₂SO₄ is present in the sample
Learn more about Avogadro's number:
https://brainly.com/question/26141731
what is the δhrxn for the cleavage of dimethyl ether using the bond energies approach?
Answers
The enthalpy change for the cleavage of dimethyl ether using the bond energies approach is 826 kJ/mol.
The cleavage of dimethyl ether (CH3OCH3) can be represented by the following equation:
CH3OCH3(g) → CH3(g) + CH3O(g)
To calculate the enthalpy change of this reaction (ΔHr), we can use the bond energies approach. This approach involves calculating the sum of the energies required to break the bonds in the reactants and the sum of the energies released by the formation of bonds in the products.
The bond energies for the relevant bonds are:
C-H bond energy = 413 kJ/mol
C-O bond energy = 360 kJ/mol
O-H bond energy = 463 kJ/mol
Using these values, we can calculate the energy required to break the bonds in the reactants:
Reactants:
4 C-H bonds × 413 kJ/mol = 1652 kJ/mol
1 C-O bond × 360 kJ/mol = 360 kJ/mol
1 O-H bond × 463 kJ/mol = 463 kJ/mol
Total energy required to break bonds in the reactants = 2475 kJ/mol
We can also calculate the energy released by the formation of bonds in the products:
Products:
2 C-H bonds × 413 kJ/mol = 826 kJ/mol
1 C-O bond × 360 kJ/mol = 360 kJ/mol
1 O-H bond × 463 kJ/mol = 463 kJ/mol
Total energy released by the formation of bonds in the products = 1649 kJ/mol
Therefore, the net energy change for the reaction is:
ΔHr = (total energy required to break bonds in the reactants) - (total energy released by the formation of bonds in the products)
= 2475 kJ/mol - 1649 kJ/mol
= 826 kJ/mol
For more question on enthalpy change click on
https://brainly.com/question/30598312
#SPJ11
For each reaction given below, write the equilibrium constant expressions K two times; one in terms of partial pressure (Kp), and other in terms of concentration (Kc).a) N2O4(g) + O3(g) <-----> 2 N2O5(g) + O2(g)b) CH4(g) + CO2(g) <-----> 2 CO(g) + 2 H2(g)c) C6H12O6(s) + 6 O2(g) <-----> 6 CO2(g) + 6 H2O(g)
Answers
An equilibrium constant is an expression that describes the relative concentrations of reactants and products at equilibrium in a chemical reaction.
Let's write the equilibrium constant expressions K for each reaction in terms of partial pressure (Kp) and concentration (Kc).
a) \(N_2O_4(g) + O_3(g) < ----- > 2 N_2O_5(g) + O_2(g)\)
\(Kp = ((P_{N_2O_5})^2 * P_{O_2}) / (P_{N_2O_4} * P_{O_3})\)
\(Kc = ([N_2O_5]^2 * [O_2]) / ([N_2O_4] * [O_3])\)
b)\(CH_4(g) + CO_2(g) < ----- > 2 CO(g) + 2 H_2(g)\)
\(Kp = ((P_{CO})^2 * (P_{H_2})^2) / (P_{CH_4} * P_{CO_2})\)
\(Kc = ([CO]^2 * [H_2]^2) / ([CH_4] * [CO_2])\)
c) \(C_6H_{12}O_6(s) + 6 O_2(g) < ----- > 6 CO_2(g) + 6 H_2O(g)\)
\(Kp = ((P_{CO_2})^6 * (P_{H_2O})^6) / (P_{O_2})^6\)
\(Kc = ([CO_2]^6 * [H_2O]^6) / [O_2]^6\)
For the last reaction, the solid reactant \(C_6H_{12}O_6\) is not included in the equilibrium expressions because its concentration remains constant throughout the reaction.
To learn more about equilibrium constant click here https://brainly.com/question/10038290
#SPJ11
(b) Using the standard reduction potentials shown in (a), show that one can prepare an ammine complex from CoCl2 and hydrogen peroxide in the presence of ammonia but not in its absence. You will need to write two redox reactions, calculate standard potentials for the reactions, and make conclusions. That is, set up an equation to calculate E°(V) using one cobalt complex half-cell with the peroxide half-cell, then calculate E°(V) again using the other cobalt complex and peroxide. Compare the two Eº values.
Answers
The E°(overall) value is higher in the presence of ammonia, we can conclude that ammonia is necessary for the formation of the ammine complex.
The two half-reactions involved in this process are:
Co2+ + 2 e- → Co E° = -0.28 V (from the table given in part (a))
H2O2 + 2 H+ + 2 e- → 2 H2O E° = 1.78 V (from the table given in part (a))
To make an ammine complex, we need to add ammonia to the reaction mixture. Ammonia can act as a ligand and coordinate with cobalt. The overall reaction can be written as follows:
CoCl2 + NH3 + H2O2 → [Co(NH3)5(H2O)]3+ + Cl- + H2O
To determine whether ammonia is necessary for the formation of the complex, we can compare the standard reduction potentials for the reaction with and without ammonia.
Without ammonia:
E°(overall) = E°(Co2+/Co) + E°(H2O2/H2O)
E°(overall) = (-0.28 V) + (1.78 V)
E°(overall) = 1.50 V
With ammonia:
E°(overall) = E°(Co3+/Co) + E°(NH3/Co3+) + E°(H2O2/H2O)
E°(overall) = (-0.49 V) + (0.76 V) + (1.78 V)
E°(overall) = 2.05 V
For such more question on ammonia:
https://brainly.com/question/14854495
#SPJ11
The E°(overall) value is higher in the presence of ammonia, we can conclude that ammonia is necessary for the formation of the ammine complex.The two half-reactions involved in this process are:Co2+ + 2 e- → Co E° = -0.28 V (from the table given in part (a))H2O2 + 2 H+ + 2 e- → 2 H2O E° = 1.78 V (from the table given in part (a))To make an ammine complex, we need to add ammonia to the reaction mixture. Ammonia can act as a ligand and coordinate with cobalt. The overall reaction can be written as follows:CoCl2 + NH3 + H2O2 → [Co(NH3)5(H2O)]3+ + Cl- + H2OTo determine whether ammonia is necessary for the formation of the complex, we can compare the standard reduction potentials for the reaction with and without ammonia.Without ammonia:E°(overall) = E°(Co2+/Co) + E°(H2O2/H2O)E°(overall) = (-0.28 V) + (1.78 V)E°(overall) = 1.50 VWith ammonia:E°(overall) = E°(Co3+/Co) + E°(NH3/Co3+) + E°(H2O2/H2O)E°(overall) = (-0.49 V) + (0.76 V) + (1.78 V)E°(overall) = 2.05 V
Learn more about determine here:
brainly.com/question/14854495
#SPJ11
what is the transformation efficiency of e coli hb101 when using the claciu chloride
Answers
The transformation efficiency of E. coli HB101 using calcium chloride can vary depending on the experimental conditions and the protocol used.
However, transformation efficiency is a measure of how many bacterial cells take up the foreign DNA and become genetically transformed, usually reported as the number of transformants per microgram of DNA. To calculate the transformation efficiency of E. coli HB101 using calcium chloride, follow the 5 steps:
1. Perform a transformation experiment using E. coli HB101 and calcium chloride. This usually involves treating the bacterial cells with calcium chloride to make them more permeable to foreign DNA, then exposing them to the DNA of interest.
2. Plate the transformed cells on selective agar plates that will allow only the transformed cells to grow.
3. Count the number of transformant colonies that appear on the selective agar plates after a suitable incubation period.
4. Determine the amount of DNA (in micrograms) used in the transformation experiment.
5. Calculate the transformation efficiency by dividing the number of transformant colonies by the amount of DNA used.
The result will be in the unit of transformants per microgram of DNA. Keep in mind that the transformation efficiency can be influenced by factors such as the quality and concentration of the DNA, the bacterial strain, and the specific experimental conditions. Therefore, the transformation efficiency for E. coli HB101 using calcium chloride may differ between experiments and laboratories.
Learn more about calcium chloride at brainly.com/question/664620
#SPJ11
Solve for the unknown M1 = 5M V1 = 30mL M2 = x V2 = 300mL A 5M B. 05M C 50M D. 5 M E 500M
Answers
Using the formula M1V1 = M2V2, the molarity of M1 = 5M, V1 = 30mL M2 = x V2 = 300mL is 0.5M. Option B is the correct answer.
To solve for the unknown in the given problem, we need to use the formula M1V1 = M2V2. This formula states that the amount of solute (Molarity) in a solution is constant, as long as the volume of the solution is constant.
We are given M1 = 5M and V1 = 30mL, and we need to find M2, given V2 = 300mL. Substituting these values into the formula, we get:
5M × 30mL = M2 × 300mL
Simplifying this equation, we get:
150 = 300M2
Dividing both sides by 300, we get:
M2 = 0.5M
Therefore, the answer is B. 0.5M, which represents the molarity of the unknown solution. In summary, we used the formula M1V1 = M2V2 and substituted the given values to find the unknown molarity, which was the solution to the problem.
Learn more about Molarity at
https://brainly.com/question/8732513
#SPJ4
.Calculate the energy released in joules/mol when one mole of polonium-214 decays according to the equation
21484 Po --> 21082 Pb + 42 He
Atomic masses: Pb-210 = 209.98284 amu,
Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
Question 8 options:
8.78 x 1014 J/mol
7.2 x 1014 J/mol
8.78 x 1011 J/mol
–9.75 x 10–3 J/mol
1.46 x 10–9 J/mol
Answers
To calculate the energy released in joules/mol when one mole of polonium-214 decays, first determine the mass difference between reactants and products: So the energy released when one mole of polonium-214 decays is 8.78 x 10¹⁴ J/mol.
To calculate the energy released in joules/mol when one mole of polonium-214 decays according to the given equation, we need to first determine the atomic mass difference between the reactants and products.
The atomic mass of 214Po is 213.99519 amu, while the combined atomic masses of 210Pb and 4He are 209.98284 amu + 4.00260 amu = 213.98544 amu.
Thus, the atomic mass difference is 213.99519 amu - 213.98544 amu = 0.00975 amu.
Using the relationship E=mc^2, we can calculate the energy released by the decay of one mole of 214Po as:
E = (0.00975 amu/mol) * (1.66054 x 10^-27 kg/amu) * (2.99792 x 10^8 m/s)^2 = 8.78 x 10^14 J/mol.
Therefore, the correct answer is 8.78 x 10^14 J/mol.
To know more about polonium-214 decays visit:
https://brainly.com/question/31859396
#SPJ11
arsenic-containing compounds such as arsenite (aso3 3- ) react readily with dithiols. arsenic compounds can covalently modify a lipoamide group. what effect would arsenic poisoning have on the activity of pyruvate dehydrogenase complex. describe the reaction step that is directly affected
Answers
Arsenic poisoning affects the activity of the pyruvate dehydrogenase complex by covalently modifying the lipoamide groups on the enzyme. This inactivates the enzyme and thus inhibits its ability to catalyze the conversion of pyruvate to acetyl-CoA.
How does arsenic poisoning affect the activity of the pyruvate dehydrogenase complex?In the process of arsenic poisoning, the arsenic compounds can modify the activity of the pyruvate dehydrogenase complex by covalently modifying a lipoamide group. Arsenic binds to sulfur in cysteine residues in many proteins, which affects protein structure and activity.
The arsenic compound reacts with dithiols and covalently modifies a lipoamide group, which directly affects the E2 subunit of the pyruvate dehydrogenase complex. E2 subunit has a lipoamide group, which acts as a cofactor, and is critical for its catalytic activity. This cofactor is also important for the interactions between the E2 subunit and other subunits of the pyruvate dehydrogenase complex. Hence, when this group is modified by arsenic compounds, it can cause irreversible inactivation of the pyruvate dehydrogenase complex. As a result, pyruvate cannot be converted to acetyl CoA, and glucose metabolism is disrupted, leading to symptoms of arsenic poisoning.
learn more about the pyruvate dehydrogenase complex
https://brainly.com/question/29997944
#SPJ11
if we start with 0.89 NaOH and we had a final weight of q.51g NaCI what is our percent yield? If it is over 100% what could be wrong with out experiment
Answers
A carbon monoxide molecule contains a _______.
A. Metallic Bond
B. Covalent Bond
C. Semisonic Bond
Answers
Answer:
B
Explanation:
What is the product of the reaction of 1-propanol with phenyl isocyanate, C6H5N=C=O?
Answers
The balanced equation for this reaction is:
CH3CH2CH2OH + C6H5N=C=O → CH3CH2CH2OC(=O)N(C6H5)CH3 + H2O
The reaction of 1-propanol (CH3CH2CH2OH) with phenyl isocyanate (C6H5N=C=O) leads to the formation of a urethane compound. The reaction's balanced equation is as follows:CH3CH2CH2OH + C6H5N=C=O → CH3CH2CH2OC(=O)N(C6H5)CH3 + H2O
In this process, the condensation reaction between the isocyanate group (-N=C=O) of phenyl isocyanate and the hydroxyl group (-OH) of 1-propanol results in the creation of a urethane molecule. A propanol group is connected to a phenyl group through an oxygen atom to produce CH3CH2CH2OC(=O)N(C6H5)CH3, the reaction's end product. The reaction also results in the production of water (H2O).Learn more about the condensation reaction:
brainly.com/question/6256866
#SPJ11
18) Based on the following equation, how many moles of hydrochloric acid are needed
to react with 0.64 moles of potassium permanganate?
2KMnO4 + 8HCI→ 3Cl₂ + 2MnO₂ + 4H₂O + 2KCI
Answers
2.56 moles of HCl are required to react with 0.64 moles of KMnO4.
The balanced chemical equation is given as;2KMnO4 + 8HCl → 3Cl2 + 2MnO2 + 4H2O + 2KCl.This equation is balanced in such a way that 2 moles of KMnO4 reacts with 8 moles of HCl to produce 3 moles of Cl2, 2 moles of MnO2, 4 moles of H2O and 2 moles of KCl.We are given the number of moles of KMnO4 as 0.64 moles.Now, we can use stoichiometry to find the number of moles of HCl required to react with 0.64 moles of KMnO4.The balanced chemical equation shows that 8 moles of HCl reacts with 2 moles of KMnO4.
So, one mole of KMnO4 would react with 8/2 = 4 moles of HCl.Now, the number of moles of HCl required to react with 0.64 moles of KMnO4 would be;Moles of HCl = Moles of KMnO4 x (Moles of HCl / Moles of KMnO4) Moles of HCl = 0.64 x 4 = 2.56 moles of HCl.
for such more questions on moles
https://brainly.com/question/15356425
#SPJ8
which chemical equations are balanced? multiple select question. c2h2 3o2 → 2co2 h2o n2 o2 → no 2na cl2 → 2nacl 4fe 3o2 → 2fe2o3
Answers
The balanced chemical equation is one that represents the same number of atoms on both the reactant and product sides of the reaction. The coefficients provide the ratios of the substances that react and form in the reaction.
The procedure for balancing a chemical equation is as follows:
Step 1: Write the unbalanced chemical equation for the reaction.
Step 2: Determine the number of atoms of each type present on the reactant and product sides of the chemical equation.
Step 3: To achieve an equal number of atoms of each element on the left and right sides of the equation, introduce coefficients.
Step 4: Verify that the chemical equation is balanced by counting the atoms of each element on each side of the equation.
Examples of balanced chemical equations are:4Fe + 3O2 → 2Fe2O3C2H2 + 3O2 → 2CO2 + H2O2Na + Cl2 → 2NaCl.
Therefore, the correct answers are 4Fe + 3O2 → 2Fe2O3, C2H2 + 3O2 → 2CO2 + H2O, and 2Na + Cl2 → 2NaCl.
Learn more about balanced chemical equations here ;
https://brainly.com/question/14072552
#SPJ11