Someone please Help please When you are measuring your pulse (heart beats per minute) what system are you monitoring?
A. Digestive
B. Respiratory
C. Circulatory
D. Integumentary (skin)
Answers
Because your check the rate of your breathing by checking the pulse
Related Questions
The balanced chemical equation for the reaction between sodium chloride and silver nitrate is: NaCl(aq) AgNO3(aq) AgCl(s) NaNO3(aq) We can interpret this to mean: ... 1 mole of sodium chloride and moles of silver nitrate React to produce ... moles of silver chloride and moles of sodium nitrate
Answers
Answer:
1 mole of NaCl reacts with 1 mole of AgNO₃ to produce 1 mole of AgCl and 1 mole of NaNO₃
Explanation:
The given reaction is a double decomposition (metathesis) reaction. A reaction in which the products are formed by the exchange of the ions present in the two reactants. NaCL and AgNO₃ exchange ions to form AgCl, which precipitates and NaNO₃.
The balanced equation for reaction is given below;
NaCl(aq) + AgNO₃(aq) ----> AgCl(s) + NaNO₃(aq)
In the reaction above, the mole ratio of the reactants to products is 1 : 1 ---> 1 : 1
This means that 1 mole of NaCl reacts with 1 mole of AgNO₃ to produce 1 mole of AgCl and 1 mole of NaNO₃.
Given the molar mass of the compounds above;
NaCl = 58.5 g/mol; AgNO₃ = 170 g/mol; AgCl = 143.5 g/mol; NaNO₃ = 85 g/mol
Therefore, 58.5 g of NaCl reacts with 170 g of AgNO₃ to produce 143.5 g of AgCl and 85 g of NaNO₃
how do people start alcohol consumption
Answers
Answer:
Explanation:Due to the mental pressure,
Due to peer pressure,
Lack of love and affection from the family members.
Influence from the T.V advertisement.
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
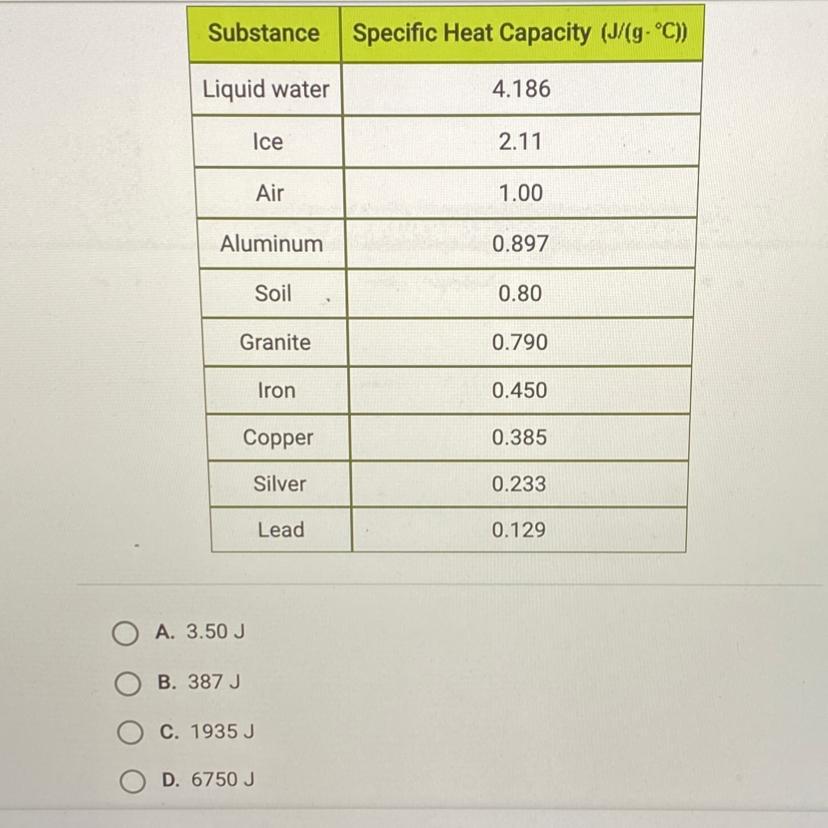
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
Calculate the temperature change of copper metal that has a mass of 43.2 g and is allowed to absorb 1,345 J of heat. The specific heat capacity of copper is 0.385 J/°c g.
Answers
Answer: 80.9 K
Explanation:
\(Q=mc \Delta T\\\\1345=(43.2)(0.385) \Delta T\\\\\Delta T=\frac{1345}{(43.2)(0.385)} \approx \boxed{80.9 \text{ K}}\)

What is SI unit of volume
Answers
Answer:
The SI unit of volume is m³
What is the density of a substance with mass of 418.23g and a volume of 436.2ml
Answers
Answer:
0.96 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume}\\\)
From the question we have
mass = 418.23 g
volume = 436.2 ml
\(density = \frac{418.23}{436 .2} \\ = 0.958803\)
We have the final answer as
0.96 g/mLHope this helps you
what is the concentration in ppm of a solution containing 0.35 mg of fluoride and 63 ml of tap water? express your answer using two significant figures.
Answers
The concentration in ppm of the solution containing 0.35 mg of fluoride is 5.56 ppm (two significant figures).
To find the concentration in ppm (parts per million) of a solution containing 0.35 mg of fluoride and 63 ml of tap water, we need to use the formula:
Concentration (ppm) = (mass of solute / volume of solution) x \(10^6\)
First, we need to convert the mass of fluoride from milligrams to grams:
0.35 mg = 0.00035 g
Next, we need to convert the volume of tap water from milliliters to liters:
63 ml = 0.063 L
Now we can plug these values into the formula:
Concentration (ppm) = (0.00035 g / 0.063 L) x \(10^6\)
Concentration (ppm) = 5.56 ppm
Therefore, the concentration in ppm of the solution is 5.56 ppm (two significant figures).
Learn more about concentration here: https://brainly.com/question/16727593
#SPJ11
How many grams of sodium hydroxide are required to prepare a 200 ml solution of a 10% (weight per volume) solution? (Atomic weights: Na = 23; 0 = 16; H = 1)
Answers
Given that we want to prepare a 10% solution of sodium hydroxide in 200 mL, we need to calculate the mass of sodium hydroxide required to make this solution.
We can use the following formula to calculate the mass of solute required to make a given volume and concentration of solution:
mass of solute = volume of solution x concentration of solution x density of soluteFirst, let's calculate the density of sodium hydroxide.The density of solid NaOH is 2.13 g/mL. So, the density of sodium hydroxide solution is:
Density = 2.13 g/mLNow, let's substitute the given values into the formula to calculate the mass of sodium hydroxide required:mass of solute = 200 mL x 0.10 x 2.13 g/mL= 4.26 gTherefore, 4.26 grams of sodium hydroxide are required to prepare a 200 mL solution of a 10% (weight per volume) solution.About Sodium hydroxideSodium hydroxide, also known as lye and caustic soda or caustic soda, is an inorganic compound with the chemical formula NaOH. This compound is an ionic compound in the form of a white solid composed of the sodium cation Na⁺ and the hydroxide anion OH⁻. Sodium hydroxide is a building block that can also be found in detergents and oil stain removers. We use it to make products clean better by influencing the formula molecules, so they work better together.
Learn More About Sodium hydroxide at https://brainly.com/question/25866669
#SPJ11
Ion ___________ have a hydrated interior that spans the membrane and allows ions to diffuse through.
Answers
Ion channels have a hydrated interior that spans the membrane and allows ions to diffuse through. These channels are integral membrane proteins that provide a selective pathway for ions to pass across the cell membrane. The hydrated interior of the channel ensures that ions remain solvated as they travel through, facilitating their movement.
Step-by-step explanation:
1. Ion channels are embedded within the cell membrane, providing a route for ions to cross.
2. The interior of these channels is hydrated, meaning that it contains water molecules, which help maintain the solvation shell around the ions.
3. This hydrated environment allows ions to diffuse, or spread, through the channel by following their concentration gradient, moving from an area of high concentration to an area of lower concentration.
4. The selective nature of ion channels ensures that only specific ions can pass through, contributing to the overall regulation of ions within the cell.
In summary, ion channels with a hydrated interior facilitate the diffusion of ions across the cell membrane, playing a crucial role in maintaining the proper balance and function of cells.
Learn more about Ion channels here: brainly.com/question/31170359
#SPJ11
To use the gas law constant R = 0. 0821, the unit for temperature should be Kelvin and the unit for volume should be milliliters.
true or false
Answers
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
define gas law constant ?
In chemistry and physics, "R" is the symbol for the gas constant, molar gas constant, ideal gas constant, or universal gas constant. In numerous equations, it is a proportionality factor that connects energy and temperature scales.
Chemistry's Gas Constant
The gas constant is also known as the ideal gas constant and the universal gas constant in chemistry.
The Boltzmann constant has a molar equivalent.
The gas constant has a SI value of 8.31446261815324 JK1mol1. The number is usually rounded to 8.314.
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
To learn more about gas constant follow the given link: https://brainly.com/question/29034664
#SPJ1
Room Temperature Is An Example Of___ a. An Attribute. b. A Variable. c. Neither Attribute Nor Variable. d. Both Attribute And Variable.
Answers
Room temperature is an example of a variable. It is not an attribute and can change based on various factors such as weather conditions, heating or cooling systems, and human activities. As a variable, room temperature can be measured and observed at different points in time, and its value can fluctuate within a certain range.
Room temperature is considered a variable because it can vary and change over time. It is not a fixed attribute of a room but rather influenced by external factors. Room temperature can be affected by the weather outside, the efficiency of heating or cooling systems, and human activities within the room. For example, during a hot summer day, the room temperature may be higher due to the heat outside, and it may decrease when an air conditioning system is turned on. Similarly, the temperature can increase when a heater is activated during cold weather. Thus, the value of room temperature is not constant and can be different at different points in time.
Moreover, room temperature can be measured and quantified using thermometers or other temperature sensing devices. It is often expressed in degrees Celsius (°C) or Fahrenheit (°F). By monitoring and recording room temperature, one can observe its fluctuations and understand its impact on comfort, energy consumption, or other relevant factors. This ability to measure and track the value of room temperature further emphasizes its variable nature.
To learn more about variable click here:
brainly.com/question/15078630
#SPJ11
A current is induced in a wire by moving the wire through a magnetic field. Which is one factor that affects the direction of the current?
Answers
Answer:
One factor that affects that affects the direction of the current is the direction of motion of the wire
Explanation:
According to Fleming's right hand rule when a conductive wire which is within a circuit is moved through a magnetic field, due to Faraday's law of electromagnetic induction an electric current is induced in the wire such that the direction of motion of the wire, the direction of the magnetic field and the direction of the electric current are perpendicular to each other such that if the right hand has the thumb middle finger and the index finger held perpendicular to each other
The motion of the wire being in the direction of the wire
The first or index finger points in the direction of the magnetic field
The middle finger points in the direction of the induced electric current
Therefore, the direction of the the current depends on the direction of motion of the wire.
NEED HELP ASAP!! 100 POINTS!!
A liter of milk has a [H+] of about 2.51 × 10–7. (You may prefer to think of the hydronium ion concentration, [H3O+], as 2.51 × 10–7.) Use the formula for the calculation of pH provided and show each step as you calculate the pH of milk. In order to get full points you must show all the steps you take.
I need the answer and all the steps.
Answers
A liter of milk has a [H+] of about 2.51 × 10–7. (You may prefer to think of the hydronium ion concentration, [H3O+], as 2.51 × 10–7.) Use the formula for the calculation of pH provided and show each step as you calculate the pH of milk. In order to get full points you must show all the steps you take.
ANSWER:
[H+] = [H3O+] = 2.52 * 10^-7M
pH = -log [H+]
pH = -log [2.51*10^-7]
pH = -[-6.60]
pH = 6.60
Hope this helps :)
The hydrogen emission spectrum is shown below. What is the energy of the
410 nm emission line? (The speed of light in a vacuum is 3.00 x 108 m/s, and
Planck's constant is 6.626 x 10-34 J.s.)
400
750 pm

Answers
Answer:
C.) 4.85 x 10⁻¹⁹ J
Explanation:
To find the energy, you need to use the following equation:
E = hc / w
In this formula,
-----> E = energy (J)
-----> h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
-----> c = speed of light (3.00 x 10⁸ m/s)
-----> w = wavelength (m)
Once you have converted nanometers to meters, you can plug the given values into the equation and solve.
410 nm 1 m
------------- x ---------------------- = 4.10 x 10⁻⁷ m
1 x 10⁹ nm
E = hc / w
E = (6.626 x 10⁻³⁴ J*s)(3.00 x 10⁸ m/s) / (4.10 x 10⁻⁷ m)
E = 4.85 x 10⁻¹⁹ J
Help me please and thanks

Answers
When an object is charged by friction, electrons are transferred from one object to another, accumulating an electric charge on each object. When an object absorbs electrons, it acquires a negative charge, while when it loses electrons, it acquires a positive charge.
A subatomic particle called an electron orbits the nucleus of an atom and has a negative charge. It weighs about \(9.11 * 10^-^3^1 kg\)and carries a charge of\(-1.602 * 10^-^1^9\) coulombs. Because they are the carriers of electric current, electrons are important for chemical reactions and the behavior of matter.
Therefore, the correct option is B.
Learn more about Electrons, here:
https://brainly.com/question/12001116
#SPJ1
consider a galvanic cell in which al3 is reduced to elemental aluminum and magnesium metal is oxidized to mg2 . write the balanced half-cell reactions that take place at the cathode and at the anode. half-cell reaction at the cathode:
Answers
The half-cell reaction at the cathode involves the reduction of Al3+ to elemental aluminum. The balanced half-cell reaction can be written as:
\(Al^{3+} + 3^{e-\) → Al
In this reaction, \(Al^{3+\) gains three electrons (e-) to become elemental aluminum (Al). The electrons are gained by the reduction half-reaction, which takes place at the cathode.
The half-cell reaction at the anode involves the oxidation of magnesium metal to Mg2+. The balanced half-cell reaction can be written as:
Mg → \(Mg^{2+ }+ 2^{e-\)
In this reaction, Mg loses two electrons (e-) to become \(Mg^{2+\). The electrons are lost by the oxidation half-reaction, which takes place at the anode.
The cathode half-cell reaction is the reduction to Al, which gains three electrons. The anode half-cell reaction is the oxidation of Mg, which loses two electrons. These half-cell reactions occur in a galvanic cell where \(Al^{3+\)ions are reduced at the cathode and Mg metal is oxidized at the anode, producing an electric potential difference between the two electrodes.
Learn more about half-cell reaction here:
https://brainly.com/question/28302447
#SPJ11
How many moles of helium are contained in 9.85 x 1023 atoms?
9.85 x 1023 atoms
Answers
Answer:
10076.55
Explanation:
How many elements are metalloids
Answers
Which one of the following statements regarding Kw is false? A) pKw is 14.00 at 25 °C. B) The value of Kw is always 1.0 × 10-14. C) Kw changes with temperature. D) The value of Kw shows that water is a weak acid.
Answers
Among the 4 given statements, option D: The value of Kw shows that water is a weak acid is the incorrect statement. Kw is ion product constant.
What is ion product constant?The ion product constant (Kw) is the product of the concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻) in aqueous solution. It is used to measure the acidity or basicity of a solution, with a Kw of 1.0 x 10⁻¹⁴ at 25 °C for pure water. The value of Kw changes with temperature and is a measure of the strength of the solvent, indicating whether it is a stronger acid or base.
The value of Kw (ion product constant for water) is a measure of the strength of the self-ionization of water, which can be represented by the equation H2O ⇌ H⁺ + OH⁻. The fact that Kw has a very small value (1.0 × 10⁻¹⁴) indicates that the self-ionization of water is a very weak reaction.
To find out more about ion product constant, visit:
https://brainly.com/question/9876097
#SPJ1
how can electronegativity cause a covalent bond to be polar
Answers
Electronegativity is the ability of an atom to attract electrons in a bond. Unequal sharing of electrons in a covalent bond occurs when atoms with different electronegativity form a bond.
When two atoms with different electronegativity values form a covalent bond, the more electronegative atom pulls the electrons closer to itself, which results in an uneven distribution of electrons in the bond. The shared electrons spend more time around the more electronegative atom, making it slightly negative, while the other atom becomes slightly positive.
The greater the difference in electronegativity, the more polar the bond will be. Polar covalent bonds are those in which the electrons are shared unequally between the atoms and result in partially charged atoms. For example, HCl molecule is polar because the chlorine atom is more electronegative than the hydrogen atom. The electrons in the bond between H and Cl are drawn more towards the chlorine, giving it a partial negative charge and the hydrogen atom a partial positive charge.
Learn more about covalent bond here:
https://brainly.com/question/19382448
#SPJ11
A 10,0-L cylinder of gas is stored at room temperature (20.0°C) and a pressure of 1800 psi. If the gas is
transferred to a 6.0-L cylinder, at what temperature in CELCIUS would it have to be stored in order for the
pressure to remain at 1800 psí? Reminder, convert your temperature to Kelvin before you begin the problem.
(Please put units)
Answers
Considering the Charles' law, the gas would have a temperature of -109.2 C.
Charles' lawFinally, Charles' law establishes the relationship between the volume and temperature of a gas sample at constant pressure. This law says that the volume is directly proportional to the temperature of the gas. That is, if the temperature increases, the volume of the gas increases, while if the temperature of the gas decreases, the volume decreases.
Charles' law is expressed mathematically as:
\(\frac{V}{T} =k\)
If you want to study two different states, an initial state 1 and a final state 2, the following is true:
\(\frac{V1}{T1} =\frac{V2}{T2}\)
Temperature of the gas in this caseIn this case, you know:
P1= 1800 psiV1= 10 LT1= 20 C= 293 K (being 0 C= 273 K)P2= 1800 psiV2= 6 LT2= ?You can see that the pressure remains constant, so you can apply Charles's law.
Replacing in the Charles's law:
\(\frac{10 L}{293 K} =\frac{6 L}{T2}\)
Solving:
\(\frac{10 L}{293 K} T2=6 L\)
\(T2=\frac{6 L}{\frac{10 L}{293 K} }\)
T2=163.8 K= -109.2 C
The gas would have a temperature of -109.2 C.
Learn more about Charles's law:
https://brainly.com/question/4147359?referrer=searchResults
an ___ filter is a device that removes oil droplets from a pneumatic system by forcing compressed air to change direction quickly.
Answers
Answer:
your answe would be a oil removal
What do you call of force acting on the ball along the horizontal line or along the that makes the ball in motion
Answers
Tension force is the force applied on the ball in a horizontal direction or along the that makes the ball in motion
Tension force is the horizontal pulling force imposed on an object by a string, rope, or cable from the opposite end. The ball is linked to a string or rope in this scenario, which is exerting tension on the ballStretching the rope or string in an attempt to bring the ball closer to it creates tension force. The ball will have a tendency to overcome the force at this point, which is why the ball will move once the force is removed.To know more about Tension force visit : https://brainly.com/question/2287912
#SPJ4
sugar dissolves in water. what is the water?
A : the solute
B : the solvent
C : the solubility
D : the solution
Answers
Answer:
The solute is the answer please select brainliest:)
Answer:
Solvent
Explanation:
Urgent!! will give brainliest!!
define an arrhenius acid and describe the properties of acids. use an example to explain how an arrhenius acid will behave when dissolved in water.
Answers
An Arrhenius acid is a substance that dissociates in water to form hydrogen ions or protons.
What is an Arrhenius acid?An Arrhenius acid increases the number of\(H^+\) ions in the water.
According to the Arrhenius theory, a substance which has hydrogen atom and can easily give hydrogen ion or proton in its aqueous solution is called as Arrhenius acid.
For example, when hydrochloric acid is dissolved in water, it forms chloride ion (\(Cl^-\)) and hydronium ion (\(H_3O^+\)).
Learn more about the Arrhenius acid here:
https://brainly.com/question/9936252
#SPJ1
is neutra
- On the basis of Rutherford's
model of an atom, which sub-
atomic particle is present in the
nucleus of an atom?
Answers
Which of the following statements is correct?
Group of answer choices
A. Low pressure indicates rising air, which allows clouds to form.
B. High pressure indicates rising air, which allows clouds to form.
C. Low pressure indicates sinking air, which allows clouds to form.
D. High pressure indicates sinking air, which allows clouds to form.
Answers
I think its A. Low pressure indicates rising air, Which allows clouds to from.
what type of bond is this combination most likely to form?
Answers
Sodium is a group 1 element with atomic number 1. It has 11 electrons. It is soft reactive metal. It has 1 valence electron.
Fluorine is a group 7 element, a hologen with 7 valence electron. It is a most reactive non metal.
When sodium react with fluorine, ionic bond is formed in the resulting compound sodium fluoride.
One sodium and fluorine each totaling 2 atoms are enough to make the bond.
As the bond is formed, both atoms have octet structure. That is they each have 8 electrons on their outermost shells.
The positive charge on sodium indicates that sodium had lost 1 electron to fluorine atom.
The negative charge on fluorine ion indicates that fluorine atom had gained 1 electron from sodium atom to form negative ion.
The name of the compound is sodium fluoride with formula NaF.
brainliest please <3
A solution is prepared by dissolving 108.3 gHCl(g) in enough water to make 135.0 L ofsolution. The pH of this solution isa. 1.66b. 12.34c. 0.096d. 2.97e. none of these
Answers
The pH of the solution prepared by dissolving 108.3 g HCl(g) in enough water to make 135.0 L of solution is (a) 1.66
To determine the pH of the given solution, we need to first calculate the concentration of H⁺ ions in the solution. HCl is a strong acid, which means it completely dissociates in water to form H⁺ and Cl- ions.
The balanced chemical equation for the dissociation of HCl is: HCl (g) → H⁺ (aq) + Cl⁻ (aq)
The molar mass of HCl is 36.46 g/mol. Therefore, the number of moles of HCl in 108.3 g is:
n = mass/molar mass = 108.3 g / 36.46 g/mol = 2.97 mol
The volume of the solution is 135.0 L, so the concentration of H⁺ ions is:
[H⁺] = n/V = 2.97 mol/135.0 L = 0.022 M
To calculate the pH, we use the equation:
pH = -log[H⁺]
Substituting the value of [H⁺], we get:
pH = -log(0.022) = 1.66
Therefore, the pH of the given solution is 1.66, which corresponds to option (a).
In summary, the given solution is prepared by dissolving 108.3 g of HCl in enough water to make 135.0 L of solution. The pH of the solution is 1.66, which is calculated based on the concentration of H⁺ ions in the solution, which is determined from the number of moles of HCl and the volume of the solution.
Learn more about pH here: https://brainly.com/question/26424076
#SPJ11
a cylindrical work bar with 5 in. diameter and 50 in. length is chucked in an engine lathe and supported at the opposite end using a live center. a 30 in. portion of the length is to be turned to a diameter of 4.6 in one pass at a speed of 153 feet per minute. the metal removal rate should be 12.53 in3/min. determine the required feed in inches/revolution at 2-decimal precision.
Answers
The required feed rate in inches/revolution for turning a 30-inch portion of the cylindrical work bar to a diameter of 4.6 inches in one pass at a speed of 153 feet per minute is approximately 0.06 inches/revolution.
To determine the required feed rate in inches/revolution, we need to use the metal removal rate and the cutting speed. The metal removal rate is given as 12.53 cubic inches per minute, and the cutting speed is given as 153 feet per minute.
First, we need to calculate the cross-sectional area of the portion being turned. Since the diameter is 4.6 inches, the radius is half of that, which is 2.3 inches. The cross-sectional area can be calculated using the formula for the area of a circle: A = πr^2. Thus, the cross-sectional area is approximately 16.619 square inches.
Next, we can calculate the required feed rate using the formula:
Feed rate = Metal removal rate / (Cross-sectional area × Cutting speed). Plugging in the values, we have Feed rate = 12.53 / (16.619 × 153). Simplifying this calculation, we find that the required feed rate is approximately 0.06 inches/revolution, rounded to two decimal places.
Therefore, the required feed rate in inches/revolution is approximately 0.06 inches/revolution.
Learn more about feed rate here:
https://brainly.com/question/13182188
#SPJ11