steric strain occurs when parts of molecules are (1) ____ and their electron clouds (2) ____ each other. molecules with steric strain are (3)_____than those without strain.options answer (select one):(1). -- Spread out. , in different directions. , too close together(2). -- bond to. , attract. , repel(3). -- more stable. , less stable , smaller
Answers
Steric strain occurs when parts of molecules are (1) too close together and their electron clouds (2) repel each other. Molecules with steric strain are (3) less stable than those without strain. Here is an explanation:
1. Parts of molecules are too close together:
Steric strain happens when atoms or groups within a molecule are forced into close proximity, leading to increased repulsion between their electron clouds.
2. Their electron clouds repel each other:
Since electron clouds are negatively charged, they naturally repel one another when forced into close proximity, creating strain in the molecule.
3. Molecules with steric strain are less stable:
The increased repulsion and strain within the molecule lead to a less stable configuration compared to a molecule without steric strain.
To learn more about steric strain refer: https://brainly.com/question/30897226
#SPJ11
Related Questions
Comparing Na and Ne: which one of these has a greater ionization energy?
Answers
Answer:
Ne
Explanation:
Comparing Ne and Na, Neon atoms will have the greatest ionization energy.
The ionization energy is the energy required to remove the most loosely held electrons within an atom.
As we go down the group, ionization energy decreases due to increase in the atomic radiusAcross a period it increases from left to right So, since Ne is right of Na, it has a higher ionization energy. It is also an inert gas and they have high ionization energyhow must the electronegativities of two atoms compare
Answers
Answer:
One atom must be much more electronegative than the other.
Calculate relative mass of chlorine and boron by the help of given data Only 30 mins are left

Answers
Jeffrey wants to lose weight by using caffeine to
help with exercise. He asks himself, "Which
caffeine drink will raise the heart rate the most?"
He tries to test this out by comparing the heart
rates of his neighbors, 3 brothers who are
triplets. One of the brothers drinks Pepsi,
another drinks a double shot of espresso (which
is coffee with twice the normal amount of
caffeine) and a third brother drinks only water for
comparison
Jeffrey finds out that the triplet with the double
shot of espresso had the highest heart rate.
4. What is the independent variable for this experiment?
5. What is the dependent variable for this experiment?
6. Which type of drink can be considered the control group? The pepsi, espresso, or water?
7 Write down 3 constants that all 3 brothers should be doing the same for this experiment?
Answers
Answer:
4. The amount of caffeine in the drinks
5. The heart rate of the participants of the experiments
6. Water
7. i) The volume of drink taken should be constant
ii) The frequency of taking their drink is constant
iii) The time of drinking by the brothers is constant
Explanation:
In the question, Jeffery intends to find the caffeine drink that will result in the heart rate increasing most
The variables (varieties) of drinks tested by Jeffery = Pepsi, espresso, and water
Drink variable arranged by the order of increasing Caffeine content are presented as follows;
Caffeine content of water < Caffeine content of Pepsi < Caffeine content of espresso
The triplet with the double shot of espresso = The triplet with the highest heart rate
4. The independent variable is the variable which is suspected to be the cause of the specified observation
Therefore, in the question, the independent variable are the drinks with different amount of caffeine
5. The dependent variable is the effect or the outcome of the independent variable
The dependent variable in the question is the heart rate of the subjects in the study
6. The control group is the independent variable or input that is expected to give the minimum effect or output compared to other independent variables in the study such that the control group does not contain the suspected cause of the observation or effect under investigation
The control group (or variable) in the question is water which does not contain caffeine
7. Three constants that all three brothers should be doing are;
i) The three brothers should be taking a constant or the same quantity of their preferred drink
ii) The frequency at which they take their drinks should be constant
iii) The time at which the brothers take the drink should be the same
Calculate the speed for a car that went a distance of 125
kilometers in 2 hours tiine.
Answers
Answer:
62.5 km/hr
Explanation:
Speed can be found by dividing the distance by the time.
s=d/t
We know the car travelled a distance of 125 kilometers in 2 hours. Therefore:
• d=125 km
• t= 2 hr
Substitute the values into the formula.
s= 125 km/ 2 hr
Divide
s= 62.5 km/ hr
The speed of the car is 62.5 kilometers per hour.
The pH of a solution can be determined using the formula pH=−log[H
+
], where H
+
is the hydrogen ion concentration in the solution. a. The hydrogen ion concentration of a particular brand of fruit juice is 0.0003 mol/L. Determine the pH of the solution, to the nearest tenth. ( 1 mark) b. A tomato has a pH of 3.0. Algebraically determine the hydrogen ion concentration of this solution. (2 marks)
Answers
(a)The pH of the fruit juice solution is approximately 3.5. (b) The hydrogen ion concentration of the tomato solution is 0.001 mol/L.
(b)The hydrogen ion concentration of the tomato solution is 0.001 mol/L.
(a). The hydrogen ion concentration of the fruit juice is 0.0003 mol/L. We can determine the pH of the solution using the formula pH = -log[H⁺].
pH = -log(0.0003)
pH ≈ -log(3 × 10⁻⁴)
Using a calculator, we can calculate the logarithm:
pH ≈ -(-3.5229) (rounded to the nearest tenth)
pH ≈ 3.5
Therefore, the pH of the fruit juice solution is approximately 3.5.
(b). A tomato has a pH of 3.0. We can determine the hydrogen ion concentration of this solution by rearranging the formula pH = -log[H⁺] to solve for [H⁺].
[H⁺] = 10(-pH)
[H⁺] = 10⁻³
[H⁺] = 0.001 mol/L
Therefore, the hydrogen ion concentration of the tomato solution is 0.001 mol/L.
To know more about hydrogen ion:
https://brainly.com/question/24673381
#SPJ4
determine the number of charged particles in nucleus of calcium atom then deduce the number of electrons
NUCLEAR CHARGE (20+)
ATOMIC MASS (40 amu)
given the relative charge of a proton =1+\ m1nucleon=1amu
Answers
Answer:
detail is given below.
Explanation:
The charged particles of nucleus are protons while neutrons are neutral having no charge.
We know that an atom consist of electrons, protons and neutrons. Neutrons and protons are present inside the nucleus while electrons are present out side the nucleus.
Electron has a negative charge and is written as e⁻. The mass of electron is 9.10938356×10⁻³¹ Kg . While mass of proton and neutron is 1.672623×10⁻²⁷Kg and 1.674929×10⁻²⁷ Kg respectively.
Symbol of proton= P⁺
Symbol of neutron= n⁰
The number of electron or number of protons are called atomic number while mass number of an atom is sum of protons and neutrons.
one proton contribute 1 amu to the total weight. There are 20 protons and 20 neutrons in Ca thus its atomic mass is 40 amu.
While the atomic number is 20.
Who and when found Neutron?
Answers
What is the oxidation number for Cl?
2
+1
-1
+2.
Answers
Answer:
-1
Explanation:
Naming Alkenes and Alkynes
Name the compounds represented by the condensed structural formulas.

Answers
1) The compound is 2-methyl but-2-ene
2) Cyclopentene
3) 2-ethyl but -1-ene
4) 2 - methyl cyclobutene
How do you name alkenes?Find the longest chain of carbon atoms that contains the double bond. This chain is the parent chain and its name is based on the number of carbon atoms it contains.
Number the carbon atoms in the parent chain so that the double bond has the lowest possible number and use the suffix "-ene" to indicate that the compound is an alkene.
If there are substituents on the parent chain, name them and indicate their location using numbers.
Learn more about alkenes:https://brainly.com/question/29283735
#SPJ1
Select the correct answer.
A circuit is used in which process?
OA. electricity
OB. magnetism
OC. velocity
OD. gravity
Answers
Answer:
electricity
Explanation:
A circuit is used in every electrical and electronics operation.
1. Balance the following equations:
(photo attached, just 1 a,b,&c)

Answers
Here are the answers
\(\\ \tt\hookrightarrow HCl+NaOH\longrightarrow NaCl+H_2O\)
Already balanced\(\\ \tt\hookrightarrow H_2SO_4+2NaOH\longrightarrow Na_2SO_4+2H_2O\)
\(\\ \tt\hookrightarrow 2HCl+Ba (OH)_2\longrightarrow BaCl_2+2H_2O\)
These pea plants have been in the sunlight since early morning.
What can the pea plants do because they are in sunlight? What does this mean for the number of energy storage molecules in the pea plants?
Answers
Pea plants uses the light energy from sun for photosynthesis and they synthesis chemical energy in leaves.
What is photosynthesis ?Photosynthesis is a biochemical process taking place in green plants to synthesis energy with the aid of light energy. The green pigments chlorophyll absorbs enough light energy for this biochemical reactions.
The photosynthetic reaction is the reaction between water and carbon dioxide producing glucose and oxygen. Thus, this process intakes carbon dioxide that we exhale and release oxygen that aids our respiration.
Pea plants are green plants undergoing photosynthesis. They uses light energy to produce chemical energy. With the presence of sunlight, they can easily carry photosynthetic reaction in leaves.
Find more on photosynthesis:
https://brainly.com/question/29764662
#SPJ1
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
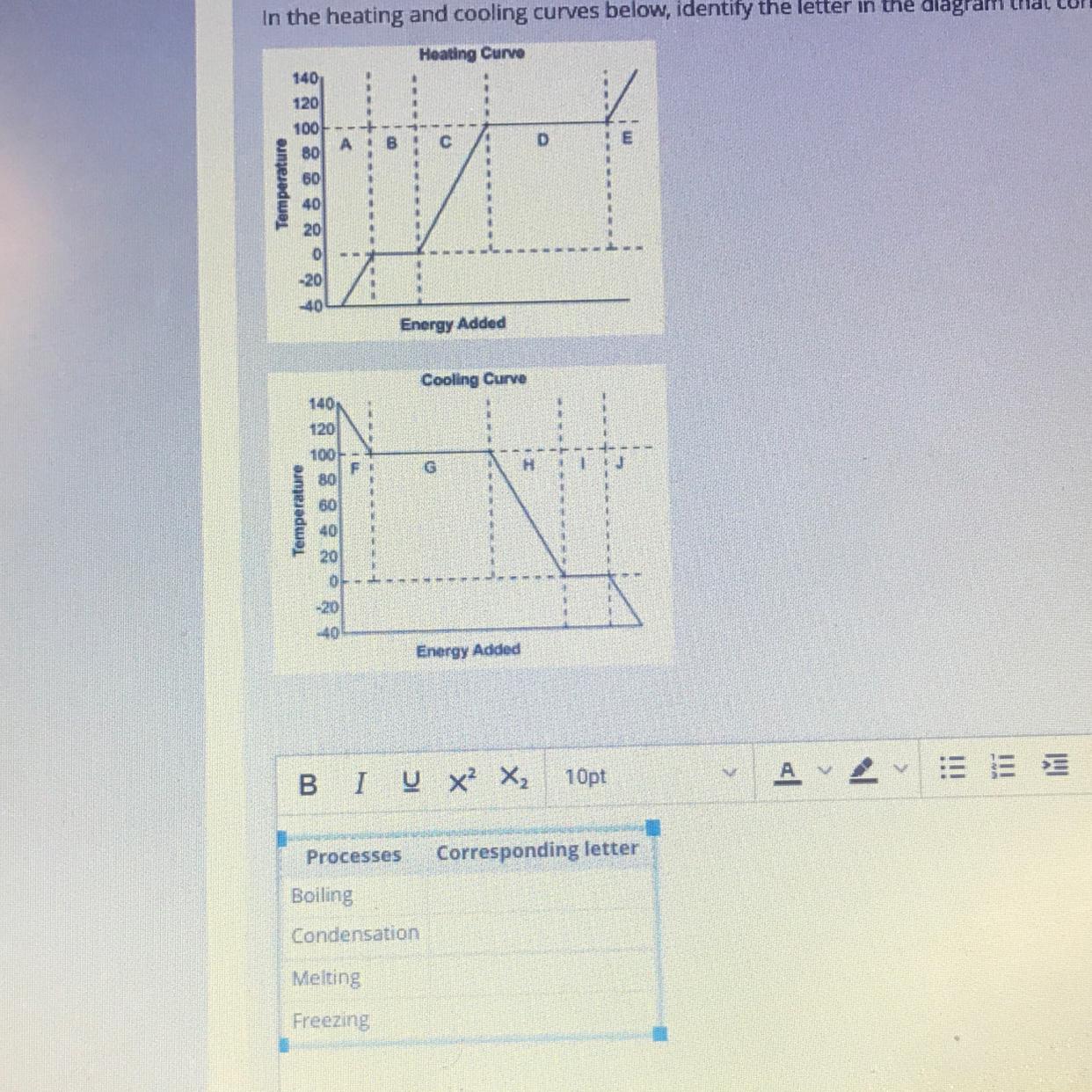
Answers
Answer:
Test for the first one is the best for
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
A bottles contains 3.100 mL of a liquid. The mass of the liquid is 2.060 g. What is the density of the liquid? (BE SURE TO
SHOW ALL THREE PARTS: EQUATION, WORK, AND PROPER FULL ANSWER.
Answers
Answer:
0.66 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{2.06}{3.10} \\ = 0.664516..\)
We have the final answer as
0.66 g/mLHope this helps you
the parts of an amino acid that are important in the protein buffer system are the
Answers
The parts of an amino acid that are important in the protein buffer system are the amino group (-NH2) and the carboxyl group (-COOH).
These two groups can act as both an acid and a base, allowing them to maintain a stable pH within the body. When the pH level in the body becomes too acidic, the amino group will act as a base and attract hydrogen ions (H+), forming NH3+.
When the pH level becomes too basic, the carboxyl group will act as an acid and release hydrogen ions, forming COO-. This ability to both accept and donate hydrogen ions makes amino acids crucial in regulating pH levels and maintaining proper physiological function.
Learn more about carboxyl group here:
brainly.com/question/31319088
#SPJ11
how can we predict if a single replacement reaction will occur
Answers
A single replacement reaction is a type of chemical reaction that takes place when one element in a compound is replaced by another element. In other words, in this reaction, one element is replaced by another element. There are a few ways to predict whether or not a single replacement reaction will occur.
These ways are explained below: Paying Attention to ReactantsThe first way to predict if a single replacement reaction will occur is by paying attention to the reactants. In a single replacement reaction, a more reactive element will replace a less reactive element. For example, if a metal element is mixed with an aqueous solution that contains ions of another metal, a single replacement reaction will occur if the metal in the solid state is more reactive than the metal in the solution state. For instance, if you put zinc in copper sulfate, a reaction will occur because zinc is more reactive than copper. Therefore, it will replace copper, producing zinc sulfate and copper.Using the Activity SeriesAnother way to predict if a single replacement reaction will occur is by using the activity series. The activity series is a list of metals and their ability to replace other metals from their compounds. This list is arranged in order of decreasing activity. Therefore, if a metal is more active than another metal on the activity series, it will replace that metal from its compound. For example, if you put magnesium in silver nitrate, a reaction will occur because magnesium is more reactive than silver. Therefore, magnesium will replace the silver, producing magnesium nitrate and silver. If the metal is less active than another metal on the activity series, no reaction will occur.Using Electrochemical SeriesThe electrochemical series is another way to predict if a single replacement reaction will occur. The electrochemical series lists the elements in order of their standard reduction potentials. A metal with a higher reduction potential will replace a metal with a lower reduction potential from its compound. For instance, if you put copper in magnesium sulfate, no reaction will occur because copper has a lower reduction potential than magnesium. Therefore, magnesium will not replace copper from its compound.
To know more about single replacement reaction visit :
brainly.com/question/29224660
#SPJ11
400 ml of a 75 M solution of H2SO4 is needed to for a lab. The stock solution is 16.0 M. Calculate how much stock is needed to make the solution.
Answers
Answer:
The volume of stock solution needed to make the solution is 1875 ml
Explanation:
The parameters given are;
The volume of 75 M solution of H₂SO₄ = 400 ml
The concentration of stock solution = 16.0 M
Number moles per liter of stock solution = 16 moles
Number of moles in required 400 ml solution = 0.4×75 = 30 M
Volume of stock solution that contains 30 M = 30/16×1 = 1.875 l
The volume of stock solution that is required = 1875 ml
zinc + nitrogen → zinc nitride
Answers
Answer:
.........................
..

Please help!!!
Perform the following operation
and express the answer in
scientific notation.
9x102: 3x1012

Answers
Answer:
3 × 10¯¹⁰
Explanation:
9×10² ÷ 3×10¹²
The above expression can be simplified as follow:
9×10² ÷ 3×10¹²
Recall:
9 = 3²
9×10² ÷ 3×10¹² = 3²×10² ÷ 3×10¹²
Recall:
a^m ÷ a^n = a^(m – n)
3²×10² ÷ 3×10¹² = 3^(2 – 1) × 10^(2 – 12)
= 3¹ × 10¯¹⁰
Recall:
a¹ = a
3¹ × 10¯¹⁰ = 3 × 10¯¹⁰
Lab: Measuring pH - Assignment: Lab Report ODL Chemistry
PLEASE HELP
100 points!!!
Answers
Here are the typical sections that you should include in your report:
Title Page - This page should contain the title of your experiment, your name, your instructor's name, and the date the experiment was conducted.
Introduction - In this section, you should provide some background information on pH and why it is important to measure it. You should also state the objectives of the experiment and describe the methodology you used.
Materials and Methods - This section should include a list of all the materials you used in the experiment, as well as a step-by-step description of the procedure you followed to measure pH. Be sure to include any safety precautions you took during the experiment.
Results - In this section, you should present the data you collected during the experiment. You should include tables, graphs, or figures to illustrate your results. You should also provide a written explanation of your findings.
Discussion - In this section, you should interpret your results and explain what they mean in terms of the objectives of the experiment. You should also discuss any sources of error that may have affected your results, and suggest ways to improve the experiment in the future.
Conclusion - In this section, you should summarize your findings and state whether or not your objectives were achieved.
References - This section should include a list of any sources you consulted during the experiment, such as textbooks, journal articles, or websites.
Appendices - This section should include any additional information that is relevant to the experiment but not included in the main body of the report, such as raw data, calculations, or photographs.
When writing your lab report, be sure to follow the formatting and citation guidelines provided by your instructor or department. You may also want to consult a scientific writing guide or other resources to help you write a clear and concise report.
\(\begin{align}\huge\colorbox{black}{\textcolor{yellow}{I hope this helps !}}\end{align}\)
\(\begin{align}\colorbox{purple}{\textcolor{lime}{Please mark as brillinest !}}\end{align}\)
\(\textcolor{cyan}{\small\textit{If you have any further questions, feel free to ask!}}\)
The mass ratio of sodium to fluorine in sodium fluoride is 1:2:1. A sample of sodium fluoride produced 28.8g of sodium upon decomposition. How much fluorine (in grams) was formed?.
Answers
Answer:
57.6g
Explanation:
1u=28.8g
2u=28.8g×2
.....=57.6g
. When identifying minerals the dilute HCI reacted with two light colored minerals. The first HCl had a strong reaction with the scratched portion of the mineral. The second mineral had only a moderate to weak reaction with the scratched surface of the mineral. What is the identity of the second mineral, or the mineral with a weaker reaction?
Answers
Based on the given information, it is possible that the mineral with the weaker reaction is a feldspar mineral, specifically an orthoclase or plagioclase feldspar.
The weaker reaction of a mineral with dilute hydrochloric acid (HCl) suggests that the mineral is likely composed of a mineral group that is less susceptible to acid dissolution. One such mineral group is the feldspar group.
Feldspar minerals, such as orthoclase and plagioclase, are commonly found in light-colored rocks and have a moderate to weak reaction with dilute HCl. They typically show a faint effervescence or no reaction at all when HCl is applied.
To know more about hydrochloric acid (HCl)
https://brainly.com/question/16683569
#SPJ11
A simplified version of the Periodic Table of the elements is provided
When moving across a row in the periodic table, which of the following increases?
Answers
Time left 1:45:17
Question 7
Not yet answered
Marked out of 3
Flag question
Question text
Consider the following reaction:
2Si2H6(g) + 7O2(g) ⇌ 4SiO2(g)+6H2O (l)
Give the expression for the equilibrium constant for this reaction. A. (PSi2H6)2(PO2)7/(PSiO2)4
B. (PSi2H6)2(PO2)7(PSiO2)4
C. (PSiO2)4/(PSi2H6)2(PO2)7
D. (PSiO2)4[(H2O])6/(PSi2H6)2(PO2)7
Answers
The equilibrium constant expression for the reaction is (PSiO2)4/(PSi2H6)2(PO2)7. Hence, the correct option is C.
In this expression, the concentrations of the reactants (Si2H6 and O2) are raised to the power of their respective stoichiometric coefficients, and the concentration of the product (SiO2) is raised to the power of its stoichiometric coefficient. The concentration of the liquid product (H2O) is not included in the equilibrium constant expression because it is in the liquid state.
The correct expression for the equilibrium constant (K) for the given reaction is:
C. (PSiO2)4/(PSi2H6)2(PO2)7
Therefore, the equilibrium constant expression for the reaction is (PSiO2)4/(PSi2H6)2(PO2)7.
Learn more about equilibrium constant from the link given below.
https://brainly.com/question/28559466
#SPJ4
A scientist distilled a quantity of water to use in an experiment. How much water did he collect if the condensation process released 9040 J of energy? H2O (l) -> H2O (g) ΔHv=2260 J/g a 0.0138 mol b 0.222 mol c 4.00 mol d 0.250 mol I really need the answer plz
Answers
Answer:
I know this is very late I am sorry, but
I believe your answer would be C) 4.00 mol.
Correct me if I am wrong.
Explanation:
9040 divided by 2260= 4
0.222 moles of water released 9040 J of energy.
The heat of vaporization is defined as the heat required to convert 1 g of a substance from liquid to vapor. We are told that the heat of vaporization of water is 2260 J/g.
Hence;
1g of water releases 2260 J of heat
xg of water releases 9040 J of heat
x = 1g × 9040 J /2260 J
x = 4 g
1 mole of water contains 18 g
x moles of water contains 4 g
x = 1 mole × 4 g/18 g
x = 0.222 moles
Learn more: https://brainly.com/question/10165688
True or false a surface wave moves the medium in the same direction as the wave travels
Answers
Answer:
true I just guessed of I'm being honest
rank the relative strength of intermolecular forces for small molecules. A. ion-dipole > hydrogen bonding > dipole-dipole > dispersion B. ion-dipole > dipole-dipole > hydrogen bonding > dispersion C. hydrogen bonding > ion-dipole > dipole-dipole > dispersion D. dispersion > dipole-dipole > hydrogen bonding > ion-dipole
Answers
Option A , Ion-dipole > hydrogen bonding > dipole-dipole > dispersion
How do intermolecular forces of attraction work?Intermolecular forces are the direct interactions that bind liquid or solid molecule together. It is a specific kind of chemical connection that takes place between molecules and is often weaker than intramolecular forces. These intermolecular forces, despite being weaker, govern some of the substances' most crucial characteristics, including boiling temperature, melting point, enthalpy of fusion, vaporization, and density. Ion-dipole > hydrogen bonding > dipole-dipole > dispersionIon-dipole interactions, which are attracted to one other by a persistent dipole, are the strongest intermolecular forces of attraction. Be aware that an ion's charge is more powerful and more persistent than a dipole's. The next step is hydrogen bonding, a unique kind of dipole-dipole interaction that results from the significant difference in electronegativity that permanently separates the charge. The remaining interactions between dipoles are weaker than hydrogen bonds. The London dispersion forces are the weakest intermolecular interactions since the dipoles are merely produced by the arbitrary movement of electrons within the bonds.
To know more about the intermolecular forces, visit:
https://brainly.com/question/9007693
#SPJ4
Which option is a natural material that is not a fossil fuel?
Natural gas
Vinyl
Petroleum
Cotton
Answers
Answer:
Cotten
Explanation:
Natural gas is fossil fuel.