The compressibility of a gas is defined by the relationship between which two measurements of the gas?
Answers
The compressibility of a gas is determined by the relationship between its volume and pressure measurements, with the compressibility factor providing a quantitative measure of this relationship and deviations from ideal gas behavior.
The compressibility of a gas is defined by the relationship between its volume and pressure measurements. Compressibility is a property that describes how easily a gas can be compressed or expanded under the influence of external pressure.
When a gas is subjected to an increase in pressure, its volume tends to decrease, indicating compressibility. On the other hand, when the pressure on a gas is reduced, its volume tends to increase, indicating expansibility. The compressibility factor (Z) is commonly used to quantify the compressibility of a gas.
Mathematically, compressibility is expressed by the equation:
Z = PV/RT,
where Z is the compressibility factor, P is the pressure, V is the volume, R is the ideal gas constant, and T is the absolute temperature.
The compressibility factor provides information about deviations from ideal gas behavior. For an ideal gas, Z equals 1 at all pressures and temperatures. However, real gases often deviate from ideal behavior due to intermolecular interactions and non-ideal conditions.
At low pressures and high temperatures, most gases behave close to ideal gas behavior and have a compressibility factor close to 1. However, at high pressures and/or low temperatures, gas molecules come closer together, and intermolecular interactions become significant, causing deviations from ideal behavior and resulting in a compressibility factor different from 1.
By studying the compressibility factor as a function of pressure and temperature, one can gain insights into the behavior of gases under various conditions, including phase transitions, critical points, and the presence of attractive or repulsive intermolecular forces.
In summary, the compressibility of a gas is determined by the relationship between its volume and pressure measurements, with the compressibility factor providing a quantitative measure of this relationship and deviations from ideal gas behavior.
learn more about compressibility here
https://brainly.com/question/32332232
#SPJ11
Related Questions
GIVING BRAINLIEST!
Please define the following in your own words:
law of conservation of matter
Answers
Answer:
No new matter can be created, and no matter can be destroyed. Matter can only change in state or chemical composition. The amount of mass before the change will equal the amount after the change.
Explanation:
do eukaryotic Cells have DNA found in the nucleus. True or false
Answers
In an ideal situation where no heat energy is produced, what is the relationship between the chemical energy provided by the battery and the electrical energy produced according to the Law of Conservation of Energy?
Answers
Answer:
See explanation
Explanation:
The principle of conservation of energy states that energy can neither be created nor destroyed but can be converted from one form to another. Hence, chemical energy in a battery can be converted to electrical energy.
Usually, the conversion of energy from one form to another is not 100% efficient according to the second law of thermodynamics. Some energy is wasted in the process, sometimes as heat.
Hence, in an ideal situation where no heat energy is produced; all the chemical energy is converted to electrical energy (100% energy conversion). There will be no energy loss if no heat is produced.
¿Cuáles son las características del átomo de carbono?
Answers
how is liming a lake similar to a doctor prescribing medicine for a patient?
Answers
Which compound contains both sigma and pi bonds? HCCl3 H2CO H2S HBr.
Answers
The compound that contains both sigma and pi bonds has been \(\rm \bold{H_2CO}\). Thus, option B is correct.
The compounds have been resulted by the sharing of the valence electrons between atoms, for the completion of octet of each elements.
The bonds can be saturated with the presence of only sigma bond. The unsaturated bonds has presence of pi bonds as well. The bond with one pi and one sigma has been a double bond, while 1 sigma and 2 pi has been a triple bond.
The bonds present in the following structures has been:
\(\rm HCCl_3=4\;\sigma\;bonds\\H_2CO=2\;\sigma,\;1\;\pi\;bond\\H_2S=2\;\sigma\;bonds\\HBr\;=1\;\sigma\;bond\)
The compound with the presence of both sigma and pi bonds has been \(\rm \bold{H_2CO}\). Thus, option B is correct.
For more information about the sigma and pi bonds, refer to the link:
https://brainly.com/question/14018074
Give the equation for the reaction of soap with HCl. What is the substance that separates from the solution when HCl is added
Answers
When HCl is added to the solution, the substance that separates from the solution is the fatty acid.
The equation for the reaction of soap with HCl is:
2RCOO^-Na^+ + 2HCl -> 2RCOOH + 2NaCl
In this reaction, soap (which is a salt of a fatty acid) reacts with hydrochloric acid (HCl) to form a fatty acid and sodium chloride (NaCl) as products.
When HCl is added to the solution, the substance that separates from the solution is the fatty acid. Fatty acids are insoluble in water and tend to separate out as a solid or a layer on top of the solution. This separation occurs because the fatty acid molecules have a long hydrocarbon chain that repels water molecules, causing them to cluster together and form a separate phase from the aqueous solution.
Thus when HCl is added to the solution, the substance that separates from the solution is the fatty acid.
learn more about fatty acid here:
https://brainly.com/question/33311830
#SPJ11
How does the mass of each atom compare to the average atomic mass of the
element given in the periodic table? *
Answers
The mass given in the periodic table is a weighted average of all isotopes of an element. For example Hydrogen is normally just one proton so one would expect its atomic mass to be exactly 1, but there are other naturally occurring isotopes of H (deuterium with a mass of 2 and tritium with a mass of 3) that have more neutrons resulting in a weighted average slightly greater than 1.
The mass of the atoms is given by the number of neutrons and protons. The mass of the atom and the average mass of the element looks a little different due to mass defect.
What is a mass defect?The mass defect has been the difference in the mass and the average mass of the elemental atom. It has been the energy that binds the neutrons and the protons together in the nucleus. The mass and the average atomic mass differ slightly.
The mass of an individual atom differs from the average atomic mass as average atomic mass is the sum of the masses of the isotope of the elements. Isotopes have little difference in masses due to the number of neutrons. For example mass and average atomic mass of hydrogen differs.
Therefore, the mass and the average atomic mass differ.
Learn more about the mass defects, here:
https://brainly.com/question/16485729
#SPJ2
As a result of the Law of Conservation of Mass, during a chemical change the mass of the
products are equal to the mass of the:
A. particles
B. elements
C. reactants
D. compounds
Answers
Answer:
D. Reactants.
Explanation:
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
bond that forms between charged ions
A.carbon-14
B.covalent
C.ionic
Answers
Answer: Ionic Bond
Explanation: They form when the cation and the anion have opposite charges, and attract each other

balance the equations

Answers
Answer: a) \(H_3PO_4+3NaOH\rightarrow Na_3PO_4+3H_2O\)
b) \(PCl_3+3H_2O\rightarrow P(OH)_3+3HCl\)
c) \(2Al+3H_2SO_4\rightarrow Al_2(SO_4)_3+3H_2\)
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side.
The balanced chemical equations are :
\(H_3PO_4+3NaOH\rightarrow Na_3PO_4+3H_2O\)
\(PCl_3+3H_2O\rightarrow P(OH)_3+3HCl\)
\(2Al+3H_2SO_4\rightarrow Al_2(SO_4)_3+3H_2\)
A 56kg skydiver jumps from the helicopter at
1524m from the ground. What is the jumper's potential energy?
Answers
The jumper's potential energy at a height of 1524 meters above the ground is equal to 836,371.2Joules.
Given the following data:
Mass of ball = 56 kilograms.Height = 1524 meters.Acceleration due to gravity = 9.8 \(m/s^2\)How to calculate potential energy.Mathematically, potential energy is given by this formula:
\(P.E = mgh\)
Where:
m is the mass.h is the height.g is acceleration due to gravity.Substituting the given parameters into the formula, we have;
\(P.E = 56 \times 9.8 \times 1524\)
P.E = 836,371.2Joules.
Read more on potential energy here: https://brainly.com/question/1242059
3. The chemical formula of a mineral can be considered a statement about the chemical components and their proportions in a mineral's structure. One of the basic tenets is that the mineral must be electrically neutral. For each of the minerals listed below, write down the mineral formulae and list the valence (oxidation) state of cations and anions that make up that mineral.
2 | Page
EASC 219: Mineralogy Fall 2022
a. uvarovite
b. azurite
c. cuprite
d. gypsum
e. galena
Answers
The valence states provided are general representations and may vary depending on specific conditions and coordination environments.
a. Uvarovite: The mineral formula for uvarovite is Ca3Cr2(SiO4)3. In this formula, the valence state of calcium (Ca) is +2, the valence state of chromium (Cr) is +3, and the valence state of silicon (Si) is +4. Oxygen (O) is usually assigned a valence state of -2.
b. Azurite: The mineral formula for azurite is Cu3(CO3)2(OH)2. In this formula, the valence state of copper (Cu) is +2, carbonate (CO3) has a valence state of -2, and hydroxide (OH) has a valence state of -1.
c. Cuprite: The mineral formula for cuprite is Cu2O. In this formula, the valence state of copper (Cu) is +1, and oxygen (O) is usually assigned a valence state of -2.
d. Gypsum: The mineral formula for gypsum is CaSO4·2H2O. In this formula, the valence state of calcium (Ca) is +2, sulfur (S) has a valence state of +6, and oxygen (O) is usually assigned a valence state of -2. The water molecules (H2O) do not have a net charge.
e. Galena: The mineral formula for galena is PbS. In this formula, the valence state of lead (Pb) is +2, and sulfur (S) has a valence state of -2.
It's important to note that the valence states provided are general representations and may vary depending on specific conditions and coordination environments.
Learn more about valence from below link
https://brainly.com/question/371590
#SPJ11
Carbonated beverages contain dissolved carbon dioxide gas. Which temperatures are best for the liquid while it is being produced in the factory?
A. High temperatures are best to minimize the solubility.
B. High temperatures are best to maximize the solubility.
C. Low temperatures are best to minimize the solubility.
D. Low temperatures are best to maximize the solubility.
Please answer and thankyou!
Answers
Carbonated beverages contain dissolved carbon dioxide gas. Low temperatures are best to minimize the solubility. option C is correct.
Drinks that have carbon dioxide dissolved in the water are referred as carbonated beverages. The presence of this gas causes the liquid to froth.
Carbonation takes place by applying pressure. Spring water, beer and soda, and pop are a few examples of carbonated beverages. When carbon dioxide is absorbed in a liquid, for example spring water, it absorbs Carbon dioxide from the subsurface. It can also happen naturally. Beer is example of a naturally carbonated beverage as the brewing process produces carbon dioxide soda .
Thus option C is correct.
To know more about carbonated beverages here
brainly.com/question/2029096
#SPJ3
INACCURATE STATEMENT: At the time of the big bang, all the matter and energy in the universe was in a tiny corner of space. Since then, it has expanded to fill up the whole universe.
Choose why this statement is inaccurate using the EVIDENCE that refutes it (proves it wrong).
1 EVIDENCE: Scientists believe the temperature of the universe immediately after the big bang was 100 billion *C. Today, the temperature of the universe is -275*C.
2 EVIDENCE: Scientists believe the very first galaxies began forming about 1 billion years after the big bang.
3 EVIDENCE: Blue light has shorter wavelengths than red light.
4 EVIDENCE: Scientists have observed galaxies are moving away from us.
5 EVIDENCE: The big bang marks the beginning of space and time.
choose only one
Answers
The evidence that refutes the statement is: 4 EVIDENCE: Scientists have observed galaxies are moving away from us.
According to the observations made by astronomers, galaxies in the universe are not only moving away from each other, but they are also moving away from us.
This phenomenon is known as the expansion of the universe, and it contradicts the idea that all matter and energy in the universe was initially concentrated in a tiny corner of space during the time of the big bang and has since filled up the entire universe.
The observation that galaxies are moving away from us suggests that the universe is expanding in all directions. This expansion implies that the universe was not initially confined to a specific location but rather underwent a rapid expansion from a highly dense and hot state.
Therefore, the idea that all matter and energy in the universe was initially concentrated in a small corner of space and then expanded to fill up the whole universe is inaccurate based on the evidence of the observed expansion of galaxies. Evidence 4
For more such questions on galaxies visit:
https://brainly.com/question/29357062
#SPJ8
HELP MEEE PLEASEEEEEEEEEEEEEE
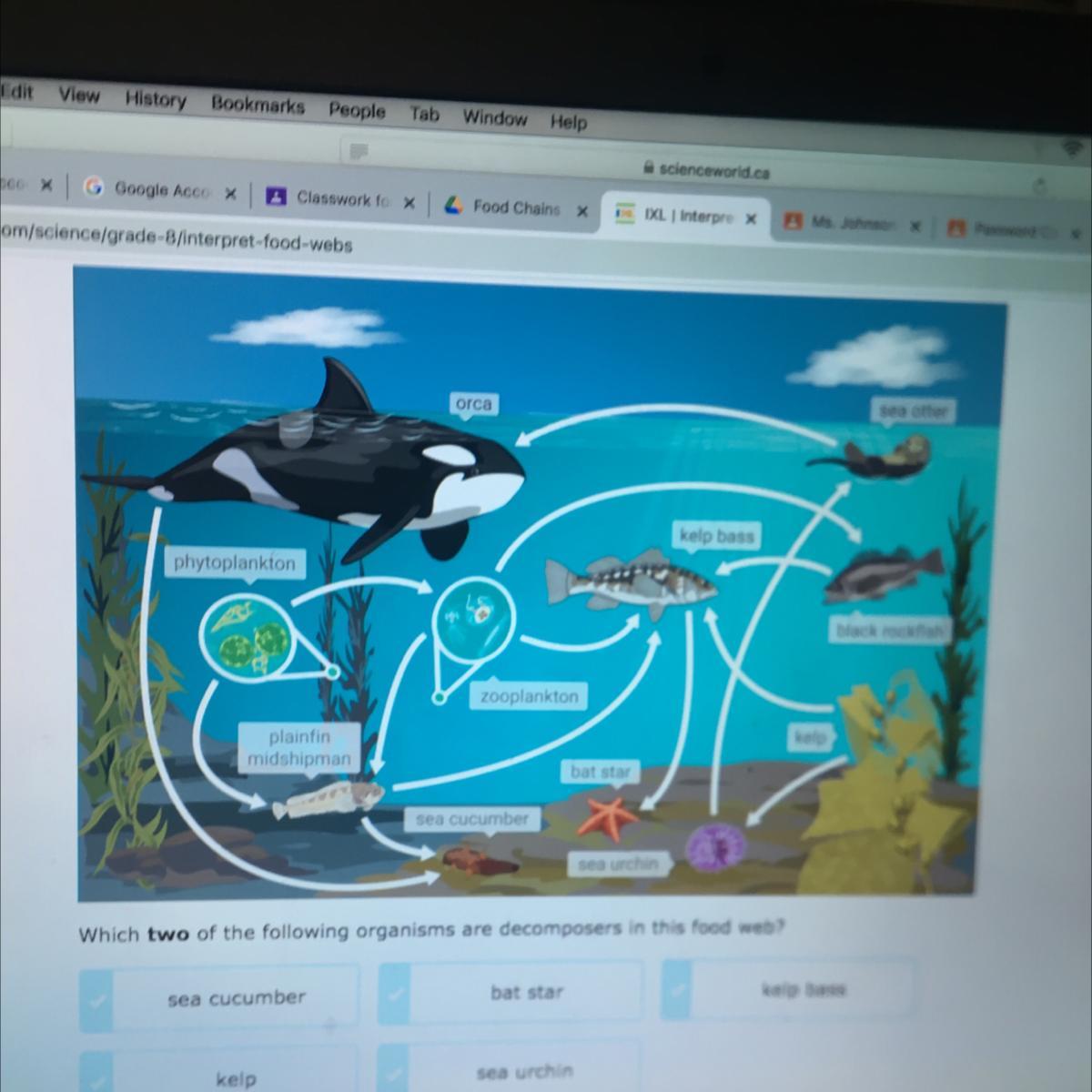
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
what is generally more efficient, extracting an organic layer using several smaller portions of water or with one large portion?
Answers
Generally, extracting an organic layer is more efficient using several smaller portions of water. This method increases the overall extraction efficiency, as each small portion of water has a higher potential to remove solutes from the organic layer compared to using one large portion.
In general, it is more efficient to extract an organic layer using several smaller portions of water instead of one large portion. This is because multiple small portions allow for a greater surface area of contact between the organic layer and water, increasing the likelihood of extracting the desired components. Additionally, smaller portions of water can be easily separated and discarded, minimizing the loss of the organic layer. Conversely, using one large portion of water may result in incomplete extraction and can also make it more difficult to separate the organic layer from the water.
Know more about organic layer here:
https://brainly.com/question/29866029
#SPJ11
The efficiency of extracting an organic layer using several smaller portions of water or with one large portion can depend on various factors such as the solubility of the organic compound in water, the volume of the organic layer, and the amount of impurities present. Using several smaller portions of water for extraction leads to more efficient removal of impurities or desired compounds from the organic layer compared to using one large portion of water.
In general, extracting with several smaller portions of water is more efficient than with one large portion. This is because using multiple smaller portions of water allows for a greater surface area of contact between the organic layer and water, increasing the chances of extracting the organic compound. Additionally, using smaller portions of water reduces the chances of emulsion formation and decreases the amount of time needed for the extraction process.
Here's a step-by-step explanation:
1. Mixing: When you mix a smaller portion of water with the organic layer, the partitioning of impurities or compounds between the two layers is more efficient, as there is a higher concentration gradient between them.
2. Separation: As the layers separate, the impurities or desired compounds transfer from the organic layer to the water layer. Using smaller portions of water multiple times increases the overall extraction efficiency.
3. Repetition: By repeating this process with several smaller portions of water, you continue to remove impurities or desired compounds from the organic layer more effectively.
In conclusion, using several smaller portions of water for extraction leads to more efficient removal of impurities or desired compounds from the organic layer compared to using one large portion of water.
For more such questions on extraction , Visit:
https://brainly.com/question/1675447
#SPJ11
Chemical mutagens that mimic the naturally occurring bases are called
a. nitrogen mustards.
b. alkylating agents.
c. base analogs.
d. nitrous oxide.
Answers
Chemical mutagens that mimic the naturally occurring bases are called Base analogs. Option C
Base analogs are a kind of mutagen that mimics the shape and size of natural nucleotide bases, allowing them to be incorporated into DNA replication. This incorporation can lead to base substitution mutations, resulting in genetic changes.
A base analog is a chemical that replaces one of the nucleotide bases in DNA and can substitute for the natural nucleotide during DNA replication. Base analogs are chemically similar to the standard nucleotide bases, allowing them to pair with the opposite base in the double helix.
The best-known base analog is 5-bromouracil, which pairs with guanine (G) in DNA replication, but also pairs with adenine (A) when it exists in its rare, unstable keto form. As a result, 5-bromouracil can cause a transition mutation when it replaces thymine (T) in DNA replication.
Base analogs are used to study DNA replication and repair mechanisms, as well as to develop cancer drugs that target rapidly dividing cells. They are also used in genetic engineering to introduce specific mutations into DNA sequences to study gene function. Option C
For more such questiosn on mutagens visit:
https://brainly.com/question/29545955
#SPJ8
All of the following features are true of primates except a prehensile tail, opposable thumbs, and the ability to swing from trees.
primates are a group of mammals that includes humans, apes, monkeys, and prosimians. They share several common features that distinguish them from other mammals. These features include:
forward-facing eyes: Primates have eyes that are positioned at the front of their face, allowing for binocular vision and depth perception.grasping hands and feet: Primates have hands and feet with opposable thumbs and toes, which allow them to grasp and manipulate objects.nails instead of claws: Primates have flat nails on their fingers and toes, rather than sharp claws.highly developed brain: Primates have a relatively large brain compared to their body size, which enables complex cognitive abilities.However, there are certain features that are not true of all primates. These exceptions include:
prehensile tail: Some primates, such as certain species of monkeys, have a tail that is adapted for grasping and can be used to hang from branches.Opposable thumbs: While many primates have opposable thumbs, not all species possess this feature. For example, prosimians like lemurs do not have opposable thumbs.ability to swing from trees: While some primates, like gibbons, are capable of swinging from tree branches using their arms, not all primates have this ability.Learn more:About primates here:
https://brainly.com/question/31118083
#SPJ11
How do i describe endothermic and exothermic changes in matter?
Answers

3.00g of glucose was burned in an excess of oxygen in bomb calorimeter with metal holder ("bomb") heat capacity of 2.21 kJ/oC. And 1.2kg of water where water has a specific heat capacity of 4.184 kJ/kgoC. The temp change upon combustion of glucose and oxygen was 19.0 0C to 25. 50C.Calculate the heat evolved from the combustion of 1.00 mol of glucose.
Answers
Answer:
THE HEAT EVOLVED FROM THE COMBUSTION OF 1 MOLE OF GLUCOSE IS - 2819.7 KJ/MOL OR -2.82 * 10^3 KJ/MOL OF HEAT.
Explanation:
Write out the variables given:
Mass of glucose = 3 g
Heat capacity of bomb calorimeter = 2.21 kJ /°C
Mass of water = 1.2 kg
Specific Heat capacity of water = 4.184 kJ/kg °C
Change in temperature = ( 25.50 °C - 19.0 °C ) = 6.5 °C
To calculate the heat evolved from the combustion of 1 mole of glucose, we do the following:
Equation for the reaction:
C6H1206 (s) + 6 02 (g)--------> 6 CO2 (g) + 6 H20 (l)
Calculate the total heat capacity involved in the system:
Heat capacity (Ctotal) = Heat capacity of the bomb calorimeter + heat capacity of water
Ctotal = 2.21 kJ/°C + (1.2 * 4.184 kJ/kg°C)
Ctotal = 7.2308 kJ°C
Next is to calculate the heat absoorbed by the calorimeter and water
Heat = Heat capacity (Ctotal) * change in temperature
Heat = 7.23 kJ/°C * 6.5 °C
Heat = 46.995 kJ
Hence, the amount of heat evolved when 3g of glucose was involved is 46.995 kJ
Since 1 mole of glucose reacts with 6 moles of oxygen to produce 6 moles of carbon dioxide and water respectively, then the amount of heat needed for the combustion of 1 mole of glucose is:
1 mole of glucose = (12 *6 + 1 * 12+ 16 *6) = 180 g/mol
From 3 g of glucose producing 46.995 kJ of heat
180 g of glucose will produce (180 * 46.995 / 3) kJ of heat
= 2819.7 kJ/mol of heat.
In conclusion, from the combustion of 1 mole of glucose, -2819.7 kJ/ mol of heat is evolved, since the heat was evolved or liberated.
why does the temperature of the reaction mixture drop (as opposed to remaining constant) once the reaction reaches the stoichiometric point? ng 5
Answers
The temperature of the reaction mixture drops once the reaction reaches the stoichiometric point due to the release of excess heat energy generated during the reaction.
During a chemical reaction, heat energy can be either released or absorbed. In an exothermic reaction, heat is released as a product, while in an endothermic reaction, heat is absorbed from the surroundings. When a reaction is not at its stoichiometric point, there is an excess of one or more reactants present. As the reaction progresses towards the stoichiometric point, the reactants are consumed, and the reactant concentration decreases.
At the stoichiometric point, the reactants are in the ideal ratio according to the balanced chemical equation. Any additional reactant beyond this point becomes excess and is no longer needed for the reaction. The excess reactant molecules do not participate in the reaction but continue to collide with each other, leading to intermolecular interactions and the release of excess heat energy. This excess heat energy dissipates into the surroundings, causing a drop in the temperature of the reaction mixture.
The decrease in temperature at the stoichiometric point is a result of the endothermic nature of the excess heat release, counteracting the exothermic nature of the reaction up to that point. This phenomenon is commonly observed in various chemical reactions and provides important insights into the energy changes occurring during the reaction process.
Learn more about reactant here: https://brainly.com/question/29035733
#SPJ11
"Asbestos needs to be removed, whether or not it will be
disturbed.
True or False"
Answers
False, Asbestos is a naturally occurring mineral fiber that was commonly used in various industries due to its heat resistance, strength, and insulating properties.
Asbestos does not necessarily need to be removed if it will not be disturbed or pose a risk to human health. Asbestos-containing materials that are in good condition and undisturbed are generally considered safe. However, if asbestos-containing materials are damaged, deteriorating, or will be disturbed during renovation or demolition activities, it is necessary to take appropriate precautions, which may include professional removal or encapsulation, to prevent the release of asbestos fibers into the air. The decision to remove asbestos should be based on an assessment of its condition, potential for disturbance, and adherence to local regulations and guidelines.
To know more about Asbestos, click here, https://brainly.com/question/8853025
#SPJ11
HELP JESUS CHRIST PLEASE ASAP

Answers
Answer:
What is a Metal ? Metals. Metals are opaque, lustrous elements that are good conductors of heat and electricity. Most metals are malleable and ductile and are, in general, denser than the other elemental substances.
Explanation:
Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties intermediate between those of a typical metal and a typical nonmetal. ...
Definition: The non-metals are elements on the right of the periodic table
C is produced as a result of combustion of organic matter with insufficient oxygen. a. Methane WA MTU M b. Benzene TO Ci Carbon dioxide Od. Carbon monoxide Oe. Mercury Om du
Answers
The correct answer is Carbon Monoxide (CO). When organic matter is burned with insufficient oxygen, it leads to incomplete combustion and the production of various gases, including carbon monoxide. Carbon monoxide is a colorless, odorless gas that is extremely poisonous and can be fatal in high concentrations.Carbon dioxide (CO2) is a product of complete combustion and is not produced when there is insufficient oxygen.Benzene is a hydrocarbon compound composed of six carbon atoms and six hydrogen atoms, and it is not produced from combustion.Methane (CH4) is a hydrocarbon compound composed of one carbon atom and four hydrogen atoms, and it is not produced from combustion.Mercury is a metallic element and is not related to combustion.
Which statement best describes how a salmon's tail fin helps it to survive?
A. It helps the salmon breathe in the water.
B. It helps the salmon keep warm in the water.
O c. It helps the salmon move through the water.
OD. It helps the salmon sense predators in the water.
Answers
Answer:
c
Explanation:
all fins are for movement
How many isomers are there in C7H16 ?
a. 6
b. 7
c. 8
d. 9
Answers
Give an example of a type of cookie that is a heterogeneous mixture, and a type of cookie that is a homogeneous mixture. Explain your reasoning.
plz answer
Answers
homogeneous- chocolate chip cookie
2. If an eagle is flying at a constant speed, it is accelerating. True or
False
Answers
Answer:
False
Explanation:
Answer:
:0
Explanation:
Part A Use your finger or a pencil to carve a riverbed into the sand, as shown in the image. The groove should be approximately 2 centimeters deep and 2 centimeters wide. Be sure that the groove does not expose the bottom of the tray. Invert and gently squeeze the water bottle so water flows into the beginning of your river. Create a steady stream, but not a flood! You'll notice that the water seeps into the sand instead of running along the top, like a river or a stream. This phenomenon also happens on Earth's surface in the water cycle. Explain which steps of the water cycle are being modeled as the water soaks into the sand.

Answers
The phenomenon observed when water seeps into the sand in the riverbed groove can be related to certain steps of the water cycle on Earth's surface.
The water cycle, also known as the hydrological cycle, involves the continuous movement of water between the Earth's surface and the atmosphere. The steps of the water cycle being modeled as the water soaks into the sand are evaporation and infiltration.
Evaporation is the process in which liquid water transforms into water vapor, primarily from the Earth's surface. In the context of the sand riverbed, as water is added to the beginning of the groove and seeps into the sand, a portion of it is likely to evaporate. The heat from the surroundings aids in the conversion of liquid water into water vapor.
Infiltration, on the other hand, refers to the movement of water from the surface into the subsurface or soil. As the water is added to the riverbed groove, it percolates through the sand, gradually infiltrating into the pores and spaces between the sand particles.
This process mimics the natural infiltration of water into the ground during rainfall or when water seeps into the soil near rivers or lakes.
By observing the seepage of water into the sand in the riverbed groove, we can draw a parallel to the water cycle's evaporation and infiltration processes.
This modeling demonstrates how water on the Earth's surface can evaporate into the atmosphere and infiltrate into the ground, replenishing groundwater reserves or feeding into natural drainage systems.
For more such questions on water cycle visit:
https://brainly.com/question/25796102
#SPJ8
is it an animal of plant cell?
Answers
Answer:
Structurally, plant and animal cells are very similar because they are both Eukaryotic cells. They both contain membrane-bound organelles such as the nucleus, mitochondria, endoplasmic reticulum, golgi apparatus, Iysosomes and peroxisomes. Plant cells can be larger than animal cells.
Explanation:
I majored in Chemistry