Answers
Answer:
C₆H₆
Explanation:
Molar mass of the compound is 78g/mol
Unknown:
Molecular formula of the compound = ?
Solution:
The empirical formula of the compound is CH.
Empirical formula is the simplest formula of a compound.
To solve this problem:
Find the molar mass of CH;
Molar mass = 12 + 1 = 13g/mol
Multiplication index = \(\frac{78}{13}\) = 6
So;
(CH)₆ ;
Therefore:
C₆H₆
Related Questions
Describes the chemical reaction (s) that produce AMD. Equations
are balanced and formatted to show subscripts.
Pls help I’m so confused
Answers
FeS2 + 7O2 + H2O → Fe2+ + 2SO4^2- + 2H+
This reaction is an oxidation reaction, where the sulfide mineral is oxidized to sulfate ions and ferrous ions are released. The ferrous ions can then react with water and oxygen to form ferric hydroxide (Fe(OH)3), which is a yellow-orange solid that contributes to the characteristic color of AMD.
The overall reaction can be written as:
4FeS2 + 15O2 + 14H2O → 4Fe(OH)3 + 8SO4^2- + 16H+
This reaction shows that four molecules of pyrite react with 15 molecules of oxygen and 14 molecules of water to produce four molecules of ferric hydroxide, eight molecules of sulfate ions, and 16 molecules of hydrogen ions. The reaction is balanced to ensure that the number of atoms of each element is the same on both sides of the equation.
Account for the low reactivity of chlorobenzene toward Ag+
Answers
The resonance structures that result form the reaction of chlorobenzene toward Ag^+ are unstable hence the low reactivity.
Why is there low reactivity?Let us recall that the kind of reaction that is going to occur here is the nucleophilic reaction. The implication of that is that the center that is going to be attacked is a center that is positive.
We now have to look at the structure of the chlorobenzene as we can see from the structures of the resonance of the compound that have been shown in the image attached.
The resonance structures that are formed by the compound are not stable and such the attacked does not lead to the production of chemical species that are stable and this is why the reactivity of the chlorobenzene towards the sliver ion is low.
Learn more about reactivity:https://brainly.com/question/1598581
#SPJ1

the density of 190mg of a liquid is 0.0076g/cm calculate the volume in ml
Answers
\(\\ \rm\Rrightarrow Density=\dfrac{Mass}{Volume}\)
\(\\ \rm\Rrightarrow Volume=\dfrac{Mass}{Density}\)
\(\\ \rm\Rrightarrow Volume=\dfrac{0.19}{0.0076}\)
\(\\ \rm\Rrightarrow Volume=25cm³\)
\(\\ \rm\Rrightarrow Volume=25mL\)
A sample of pure copper has a volume of 3.75 cm3. Calculate its mass
Answers
Answer:
33.60 g
Explanation:
In order to solve this problem we need to know the density of pure copper. That value can be found on the periodic table or in textbooks: 8.96 g/cm³.
Knowing the density of copper, we can calculate the mass of a sample that occupies 3.75 cm³:
Density = mass / volumeMass = Density * volume8.96 g/cm³ * 3.75 cm³ = 33.60 gA student sets up the investigation shown below. The movement of colored water through the gravel best models —
Answers
A student sets up the investigation shown below. The movement of colored water through the gravel best models groundwater.
What is a groundwater?Groundwater is water that is located beneath the surface of the Earth in soil pore spaces and in the fractures of rock formations. It originates from precipitation that percolates into the soil and moves downward to the water table, which is the boundary between the unsaturated and saturated zones of soil.
Groundwater can be a major source of drinking water and is also used for irrigation, industry, and other purposes. It can be accessed through wells drilled into the ground, and it often moves very slowly through the subsurface, sometimes taking decades or even centuries to replenish.
Find out more on groundwater here: https://brainly.com/question/10557415
#SPJ1
The complete question:
A student sets up the investigation shown below. The movement of colored water through the gravel best models —

How Can I prepare Conc. of Ethanol in water 25% …?
Answers
Answer:
25 percent solution by mass of ethanol means that 100 gram of solution contains 25 gram of solute i.e-ethanol. Now,as the density of the solution is 1 g/ml so 100 g of the solution has a volume of 100 ml. So,we have 25 gram of ethanol in 100 ml of the solution. So we have 250 g of ethanol in 1 litre of solution.
Explanation:
The photograph shows the most abundant substance on Earth's su Which fact about this substance tells you that it is a compound? A. It is a colorless liquid that is inflammable. B. It is made up of atoms that have mass and take up space c. It can be separated into two substances by a chemical pro D. It appears to be the same type of matter throughout.
Answers
Answer:
the answer to this question is b
Name the piece of equipment shown above:

Answers
Answer
a graduated cylinder! graduated cylinder’s provide more accurate and specific measurements than beakers do!
Explanation:
List four kinds of energy. Give a brief definition of each.
Answers
Answer:
Mechanical Energy- Energy that result from movement or the location of the object. Is the sum of kinetic and potential energy.
Thermal Energy- Thermal energy or heat energy reflects the temperature difference between two systems.
Nuclear Energy- is energy resulting from changes in the atomic nuclei or from nuclear reactions.
Chemical Energy- results from chemical reactions between atoms or molecules.
More:
Kinetic energy- is the energy of motion of a body. It ranges from 0 to a positive value.
Electromagnetic energy- (or radiant energy) is energy from light or electromagnetic waves.
Sonic energy- is the energy of sound waves. Sound waves travel through the air or another medium.
Gravitational energy- energy associated with gravity involves the attraction between two objects based on their mass.
Ionization energy- is the form of energy that binds electrons to the nucleus of its atom, ion, or molecule.
Potential energy- is the energy of an object's position.
Explanation:
Hope this helps
Calculate the root main Square Velocity for the atoms in a sample of Nitrogen gas (N2) at 25°c of CO₂ and SO₂ from the same container and at the same temperature and pressure.
Answers
Answer:
To calculate the root mean square velocity of atoms in a gas, we need to know the mass of the atoms, the temperature of the gas, and the gas constant. The root mean square velocity can be calculated using the following equation:
v_rms = sqrt((3RT)/m)
Where v_rms is the root mean square velocity, R is the gas constant, T is the temperature in kelvins, and m is the mass of the atoms in kilograms.
For nitrogen gas (N2), the mass of each atom is 14/6.022 x 10^23 = 2.34 x 10^-23 kg.
For CO2, the mass of each atom is 12/6.022 x 10^23 = 2.0 x 10^-23 kg.
For SO2, the mass of each atom is 32/6.022 x 10^23 = 5.3 x 10^-23 kg.
Plugging these values into the equation, we can calculate the root mean square velocity for each gas at 25°C:
v_rms (N2) = sqrt((38.31298)/(2.34 x 10^-23)) = 466 m/s
v_rms (CO2) = sqrt((38.31298)/(2.0 x 10^-23)) = 505 m/s
v_rms (SO2) = sqrt((38.31298)/(5.3 x 10^-23)) = 691 m/s
Therefore, the root mean square velocity of atoms in a sample of Nitrogen gas at 25°C is 466 m/s, while the root mean square velocity of atoms in a sample of CO2 at the same temperature and pressure is 505 m/s, and the root mean square velocity of atoms in a sample of SO2 at the same temperature and pressure is 691 m/s.
Explanation:
SELF EXPLANATORY
Which statement about the relationship between laws, hypotheses, and theories is true?
Answers
HELP I DON'T HAVE LONG LEFT AND I'M STRUGGLING SO BAD PLEASE I BEG U TO HELP

Answers
5. Which ion has the same electron configuration as argon (Ar):
o S2-
O A13+
Ba2+
Answers
Answer:
o s2- or o A13 + Ba2+ or something else
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Which units can be used to describe the thermal
energy within a substance?
Answers
Answer: Btu, British Thermal Unit is used to express thermal energy when working in pounds and degrees Farenheit.
Calories are used when working in grams and degrees Centigrade or Kelvin (SI units)
A Joule is the British system unit used to measure heat transfer.
Explanation:
The difference between voltaic and electrolytic cells is that:
Answers
Answer:
Voltaic cells convert chemical energy to electrical energy by means of an oxidation-reduction reaction. Electrolytic cells convert electrical energy to chemical energy, so they are the opposite of voltaic cells. They require an input of electrical energy to cause an oxidation-reduction reaction.
Explanation:
that good enough for ya my asian friend?
Answer:
This is the answer hopefully you understand this also can you mark me brainliest pls

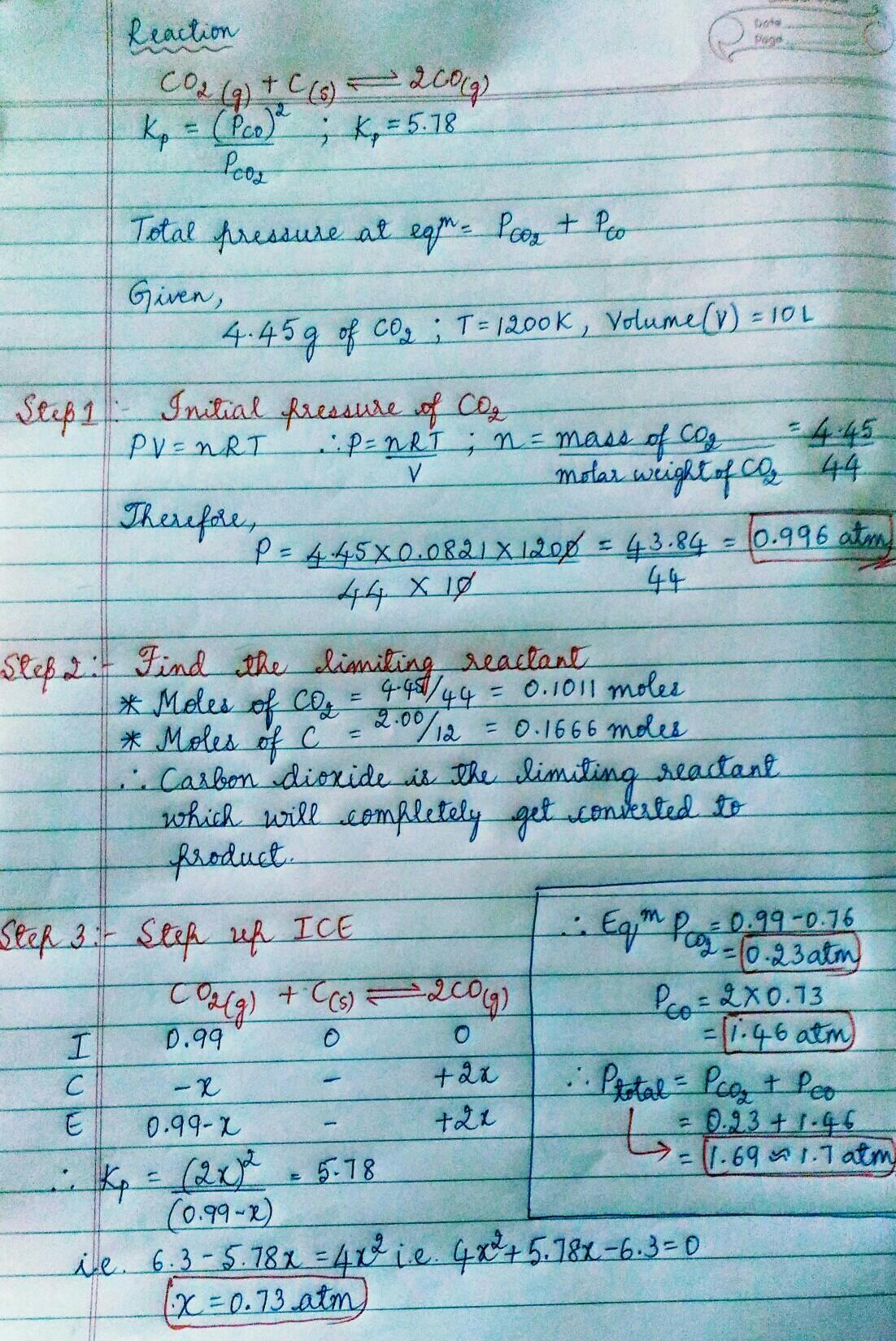
The reaction CO₂(g) + C(s) = 2 CO(g) has Kp = 5.78 at
1200 K.
a. Calculate the total pressure at equilibrium when 4.45 g of CO₂ is introduced into a 10.0-L container and heated to 1200 K in the presence of 2.00 g of graphite.
b. Repeat the calculation of part a in the presence of 0.50 g of graphite.
Answers
Hope this answer helps you a lot ✅✅
how might volcanic eruptions disrupt earth energy budget
Answers
Answer: large volcanic eruptions inject light-reflecting particles as high as the stratosphere.
Explanation:
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
When a substance breaks up into two simpler substances, the reaction
is an)
reaction.
Answers
Answer:
Decomposition.
Explanation:
Decomposition Reactions T hose reactions in which a single substance (reactant) splits up into two or more simpler substances (products) are known as decomposition reactions. These reactions are carried out by supplying energy in form of heat, electricity or light which breaks that substance into simpler substances
Please Help ASAP!!
100 pts + Brainliest!!

Answers
Answer:
Hope this helps ;) don't forget to rate this answer !
Explanation:
The molar mass of a sample can be calculated using the formula:
Molar mass = (molecular weight) / (number of moles)
To find the molar mass, we first need to find the molecular weight of the sample. The molecular weight is the weight of a single molecule of the substance in atomic mass units (amu).
To find the molecular weight, we need to convert the weight of the molecule from grams to amu. One amu is equal to 1.66 x 10^-24 grams.
So, to convert the weight of the molecule from grams to amu, we can divide the weight of the molecule (5.34 x 10^-23 grams) by the conversion factor (1.66 x 10^-24 grams/amu):
Molecular weight (amu) = (5.34 x 10^-23 grams) / (1.66 x 10^-24 grams/amu)
= 3.22 x 10^-22 amu
Now that we have the molecular weight, we can use it to calculate the molar mass. However, we need to know the number of moles in order to do this. Without this information, it is not possible to calculate the molar mass.
Answer:
i agree
Explanation:
write an equation for the proton transfer reaction that occurs when the following base reacts with water. draw curved arrows that show a mechanism for the proton transfer, and modify the given structures to draw the resulting products.
Answers
Refer the product in the image.The transfer of a proton (H+) in chemistry typically occurs through a mechanism known as an acid-base reaction.
This process involves the transfer of a proton from an acidic species (the proton donor) to a basic species (the proton acceptor), leading to the formation of a new acidic species and a new acid-base reactions species. In the final structure, the tetrahedral shape of the molecule and the absence of charge indicate that the proton transfer has resulted in the formation of a neutral species. In other words, the proton has been transferred from an acidic species to a basic species, neutralizing both species and resulting in a neutral compound with a tetrahedral shape. However, the general idea of the transfer of a proton from an acidic species to a basic species remains a key principle in understanding acid-base reactions.
Learn more about acid-base reactions here:
https://brainly.com/question/10467673
#SPJ4
Refer the below image to answer the Question:


which probing question lies within the scope of physics?
Answers
Physics is a vast field that addresses a wide range of questions about the nature of the physical world. Probing questions can help to explore this field and encourage critical thinking and deep exploration of its topics.
Probing questions are open-ended questions asked to gather information, encourage critical thinking and deep exploration of a particular topic. Physics is a natural science that studies matter and its motion through space-time. It is a branch of science that deals with the fundamental nature of the universe and seeks to explain how and why objects behave as they do in the physical world.The following are some examples of probing questions within the scope of physics:What is the nature of light-The nature of light is an important topic within the scope of physics. It refers to the dual nature of light, as both a wave and a particle. Light behaves as a wave when it is traveling through space and as a particle when it is interacting with matter.How do magnets work-Magnets are a common object in the world around us, and they have a broad range of applications. They work by producing a magnetic field, which can attract or repel other magnetic objects. This topic lies within the scope of physics.What is the relationship between energy and matter-Energy and matter are two fundamental concepts in physics. The relationship between them is described by Einstein's famous equation E=mc2, which states that matter and energy are two forms of the same thing and are interchangeable. The study of the relationship between energy and matter lies within the scope of physics.What is the nature of the universe?The study of the universe's nature is one of the most significant topics within the scope of physics. This question addresses the origins and properties of the universe, its components, and the laws that govern its behavior.
for such more questions on physical
https://brainly.com/question/1079154
#SPJ8
What will increased temperature in a reaction cause
1 Particles in a reaction to move more slowly
2 Particles in a reaction to move faster
3 Particles in a reaction to generate more electrical ions
4 Particles in a reaction to lose more electrical ions
Answers
When 7.524 is rounded to 3 sig figs it will be
Answers
When 7.524 is rounded to 3 significant figures, it will be 7.52.
The process of changing a number to a nearby number with fewer significant digits is known as rounding.
Rounding can be done to the nearest integer, the nearest tenth, the nearest hundredth, and so on.
Here are some pointers on rounding numbers to a certain number of significant digits:If the digit following the last significant digit is less than 5, simply drop it and all following digits.
(round down)For example, 2.832 rounded to two significant digits is 2.8 since the 3 is followed by a 2 which is less than 5.
If the digit following the last significant digit is greater than 5, add 1 to the last significant digit, then drop all of the digits that follow it.
(round up)For example, 4.673 rounded to two significant digits is 4.7 since the 3 is followed by a 7 which is greater than 5.
If the digit following the last significant digit is exactly 5, the preceding digit is odd, and no other digits follow, increase the last significant digit by 1.
If the digit following the last significant digit is exactly 5, the preceding digit is even, and no other digits follow, simply leave the last significant digit alone.
For example, 2.875 rounded to two significant digits is 2.9 since the 5 is followed by an odd number, which means that the 8 should be rounded up, while 2.765 rounded to two significant digits is 2.8 since the 5 is followed by an even number, which means that the 6 should be left alone.
For more such questions on rounding
https://brainly.com/question/17396482
#SPJ8
What is the answer, and how to solve this equation

Answers
The combustion reaction of diborane (B2H6) with oxygen (O2) is:
B2H6(g) + 3 O2(l) -> 2 HBO2(g) + 2 H2O(l)
We are given that 198.4 g of B2H6 is used, and we want to find the mass of liquid oxygen (LOX) required for the reaction.
From the balanced equation, we can see that for every 1 mole of B2H6, 3 moles of O2 are needed.
We can use the molar mass of B2H6 (37.83 g/mol) to convert the mass of B2H6 to moles:
198.4 g / 37.83 g/mol = 5.24 moles B2H6
Since 3 moles of O2 are needed for every 1 mole of B2H6, we can multiply the number of moles of B2H6 by 3 to find the number of moles of O2 required:
5.24 moles B2H6 x 3 moles O2/1 mole B2H6 = 15.72 moles O2
We can use the molar mass of O2 (32.00 g/mol) to convert the number of moles of O2 to mass:
15.72 moles O2 x 32.00 g/mol = 502.24 g
So, the mass of liquid oxygen (LOX) needed to burn 198.4 g of B2H6 is approximately 502.24 g.
002.
The combustion reaction of diborane (B2H6) with oxygen (O2) is:
B2H6(g) + 3 O2(l) -> 2 HBO2(g) + 2 H2O(l)
We are given that 126.1 g of B2H6 is used, and we want to find the mass of HBO2 produced from the combustion.
From the balanced equation, we can see that for every 1 mole of B2H6, 2 moles of HBO2 are produced.
We can use the molar mass of B2H6 (37.83 g/mol) to convert the mass of B2H6 to moles:
126.1 g / 37.83 g/mol = 3.34 moles B2H6
Since 2 moles of HBO2 are produced for every 1 mole of B2H6, we can multiply the number of moles of B2H6 by 2 to find the number of moles of HBO2 produced:
3.34 moles B2H6 x 2 moles HBO2/1 mole B2H6 = 6.68 moles HBO2
We can use the molar mass of HBO2 (48.05 g/mol) to convert the number of moles of HBO2 to mass:
6.68 moles HBO2 x 48.05 g/mol = 319.55 g
So, the mass of HBO2 produced from the combustion of 126.1 g of B2H6 is approximately 319.55 g.
How does Boron achieve a noble gas electron configuration?
Answers
Boron gas can achieve noble gas configuration by losing or gaining electrons.
Noble gases were substances that have completed respective octets and have entirely filled respective valence shells. Helium (He), Neon(Ne), Argon(Ar), Krypton(Kr), Xenon (Xe), as well as Radon, are still examples of noble gases.
Atoms can adopt a noble gas configuration whether by acquiring new electrons or by giving and receiving electrons already present in even their own outermost shell.
The atomic number of Boron is 5 . the electronic configuration of B is 2,3. When it will gain 5 electrons with another atom or lose 3 electrons then it will attain a noble gas configuration.
To know more noble gas configuration
https://brainly.com/question/27848059
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Why do we study chemistry?
Answers
Answer:
The study of chemistry provides global work opportunities.
Explanation:
Answer:
2 answers.
Explanation:
1. The study of chemistry provides global work opportunities. Chemistry underpins understanding and progress in almost every science, technology, and industry sphere. It also makes a vital contribution to the economy, commerce and industry.
2. Because teachers want us to be bored and make time feel like it's stuck in honey.