the half cell that is normally chosen to have a potential of zero is
Answers
The half cell that is normally chosen to have a potential of zero is the standard hydrogen electrode (SHE).
The standard hydrogen electrode consists of a platinum electrode in contact with a solution of hydrogen ions at a concentration of 1 mol/L and a pressure of 1 atm of hydrogen gas. The electrode potential of the SHE is defined as 0 V at all temperatures. Other half cells are compared to the SHE to determine their electrode potentials, which can be positive or negative relative to the SHE.
The choice of the SHE as the reference electrode is based on its reproducibility and stability, as well as the fact that hydrogen ions and hydrogen gas are present in many electrochemical reactions. Using the SHE as the reference allows for accurate comparisons of electrode potentials and standardization of electrochemical measurements.
In summary, the half cell that is normally chosen to have a potential of zero is the standard hydrogen electrode, which serves as a reference electrode for electrochemical measurements.
Learn more about potential here:
https://brainly.com/question/4305583
#SPJ11
Related Questions
Which statements are true of heterogeneous mixtures? check all that apply. they settle out. they exhibit the tyndall effect. their solutes and solvents appear as one. they exhibit brownian motion. they are evenly distributed mixtures.
Answers
The statements which are true about heterogeneous mixtures are they settle out, they exhibit the tyndall effect and they exhibit brownian motion.
What are heterogeneous mixtures?Heterogeneous mixtures are those mixtures in which composition of their constituent partiles are not identical or even, they may vary place to place.
In the heterogeneous mixtures, solutes are present unevenly in the solution and this uneven arrangement is also arises the brownian motion means the randon motion of the particles. And these mixtures also shows the tyndall effect as light passes through the suspended particles may get scattered. On placing these mixtures without any motion for a long time, then it may sometimes settle down at the bottom.Hence, options (1), (2) & (4) are correct.
To know more about heterogeneous mixtures, visit the below link:
https://brainly.com/question/1080253
Answer:
A,B,D
Explanation:
i got it right
Which of these is Not a sign that a chemical change has occurred?
A. a loss of transparency
B. a temperature change
C. an unexpected color change
D. The formation of a precipitate
Answers
Answer: c
Explanation:
An unexpected color change is not a sign that a chemical change has occur. Thus option c is correct.
What is chemical change?A chemical change is defined as the transformation of one material into another, the formation of new materials with different properties, and the formation of one or more new substances.
There are basically five types of chemical reaction.
Combination reactionDecomposition reactionDisplacement reactionDouble displacement reactionSynthesis reactionThus, an unexpected color change is not a sign that a chemical change has occur. Thus option c is correct.
To learn more about chemical change, refer to the link below:
https://brainly.com/question/8159283
#SPJ2
Write an experiment to show the process of rusting
Answers
need help with this as soon as possible
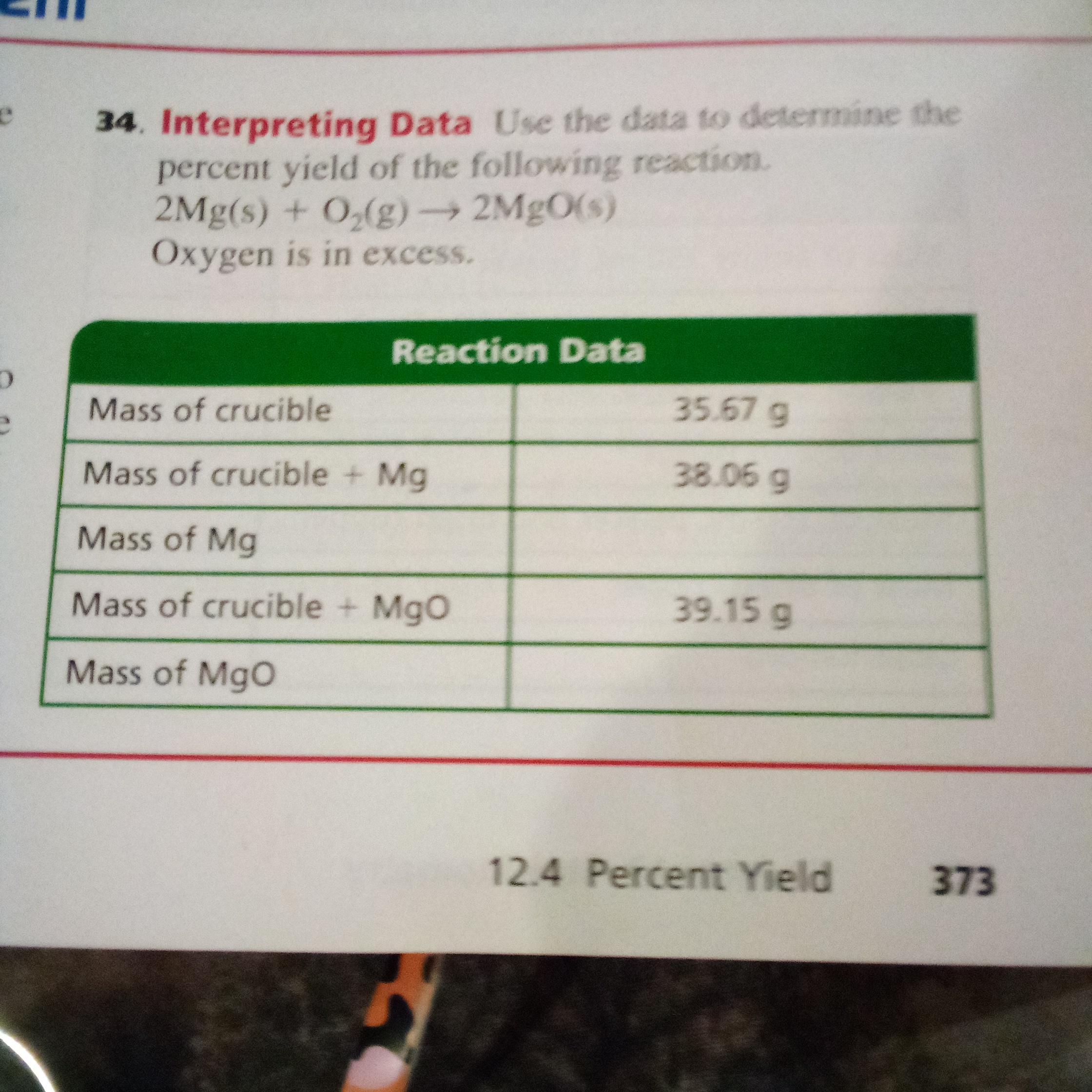
Answers
Mass of Mg is 2.39g and Mass of MgO is 3.48 g. Mg is a chemical element Magnesium.
How to calculate mass ?The average mass of an element's atoms expressed in atomic mass units is the element's atomic mass. The mass of each isotope is multiplied by its abundance to produce the atomic mass, which is a weighted average of all the isotopes of that element.
The atomic mass of an element, measured in amu, is equal to the mass in grammes of one mole of an element. This relationship between the atomic mass and the mole concept is important in chemistry.
Mass of Mg = Mass of crucible and Mg - Mass of empty crucible = 38.06g - 35.67g = 2.39g
Mass of MgO = 39.15 g - 35.67 g = 3.48 g
To learn more about atomic mass refer :
https://brainly.com/question/3187640
#SPJ1
initial consentretion
Answers
What is initial concentration?
High initial concentration of spilled oil has a negative effect on the biotransformation process that causes a significant lag phase of about 2–4 weeks. Even after biostimulation, at least a week is needed for microorganisms to adapt and the entire bioremediation process may require months and even years to be completed (Atlas, 1995; Zahed et al., 2010a). The rates of uptake and mineralization of many organic compounds by a microbial population depend on the concentration of the compound (Olivera et al., 1997; Rahman et al., 2002).
Step by step explanation
Why is an occluded front considered the most complex type of front?
Answers
Answer:
folloow mw tiktokjonathan_dion21 for a free cookie
Explanation:
Nitrogen gas (N2) and hydrogen gas (H2) react to produce ammonia (NH3).
If we have 2 mol of N2, how many moles of NH3 will be produced?
1 mol NH3
2 mol NH3
3 mol NH3
4 mol NH3
Answers
Answer:
I believe it would be 2
Explanation:
if there are 2 nitrogen molecules then there would have to be 2 hydrogen molecules but this could be completely wrong
if this happens to be right, ur welcome :3 here's a Jay Jay pic for u cuz why not

Answer:
Anwer is A 0.60
Explanation:
This molecule has ___ bonds and is a ___ molecule.
a. nonpolar, polar
b. polar, nonpolar
c. nonpolar, nonpolar
d. polar, polar
explain why

Answers
what is the empirical formula of a compound composed of 3.25 hydrogen 19.36 carbon
Answers
Answer:
The empirical formula of the compound is CH2.
Explanation:
To determine the empirical formula of a compound, we need to find the simplest whole-number ratio of atoms present in the compound. Here, the percentage composition of hydrogen (H) and carbon (C) in the compound, we can calculate the empirical formula.
Here, Percentage of hydrogen (H) = 3.25%
Percentage of carbon (C) = 19.36%
Convert the percentages to grams.
Assume we have 100 grams of the compound, which allows us to directly convert the percentages to grams.
Mass of hydrogen (H) = 3.25 g
Mass of carbon (C) = 19.36 g
Convert the masses to moles.
To convert grams to moles, we need to divide the masses by the molar masses of hydrogen and carbon.
Molar mass of hydrogen (H) = 1.008 g/mol
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of hydrogen (H) = Mass of hydrogen / Molar mass of hydrogen
Number of moles of hydrogen (H) = 3.25 g / 1.008 g/mol ≈ 3.22 mol
Number of moles of carbon (C) = Mass of carbon / Molar mass of carbon
Number of moles of carbon (C) = 19.36 g / 12.01 g/mol ≈ 1.61 mol
Determine the empirical formula.
Divide the number of moles by the smallest number of moles obtained.
Dividing the moles of carbon (1.61 mol) by the smallest value (1.61 mol) gives 1.00.
Dividing the moles of hydrogen (3.22 mol) by the smallest value (1.61 mol) gives 2.00.
The simplest whole-number ratio of atoms is approximately C1H2
Therefore, the empirical formula of the compound is CH2.
Learn more about empirical formula here, https://brainly.com/question/1603500
#SPJ11
waves are blank that transfer blank through matter or blank
Answers
Waves are disturbance that transfer energy through matter or space.
A wave can be described as a disturbance that travels through a medium, transporting energy from one location to another location without transporting matter.
The particles involved in waves move back and forth perpendicularly to the way the wave is going, but don’t move significantly in the direction of the wave. The particles ‘take part’ in the wave by bumping into one another and transferring energy. This is why energy can be transferred, even though the average position of the particles doesn’t change.
Learn more about Waves, here:
https://brainly.com/question/25954805
#SPJ1
Please help with these two (last question is nwse)

Answers
Answer:
your answer gonna be 20 miles
what do calories have to do with combustion
Answers
Answer:
A calorie is the amount of energy needed to raise the temperature of 1 gram of water 1 degree Celsius. The complete combustion of a large kitchen match, for example, gives you about one kilocalorie of heat
1.) You have a sample of 1.64 moles of aluminum carbonate is mixed with lithium to produce lithium carbonate and aluminum. How many moles of lithium would completely react with all the aluminum carbonate.
2.) a synthesis reaction between magnesium and nitrogen forms an ionic compound magnesium nitride. If you have 4.226 moles of magnesium how many grams of nitrogen will completely react with the magnesium.
3.) given the following equation 8Fe+S8>8FeS, how many grams FeS are produced, and what mass of iron is needed to react with 16 grams of sulfur
4.) B2H6+3O2>HBO2+2H2O, what mass of O2 will be needed to burn 31.6g B2H6, and how many miles of water are produced from 12.8g B2H6
Answers
Lithium carbonate contains 18.78% mass percent lithium. It should be noted that a compound's overall percentage composition of all its constituent elements is always 100%.
Is lithium carbonate a depressive medication?Only depression related to bipolar illness is authorized for lithium use. When combined with an antidepressant, it may also be successful in alleviating other types of depression, although further research is required. Discuss the possibility of adding lithium with your doctor if you are on an antidepressant but are still experiencing symptoms.
What purpose does lithium carbonate serve?This substance is employed for the treatment of mania and depression (bipolar disorder). By bringing certain natural compounds back into equilibrium in the brain, it helps to calm mood and lessen excessive behavior.
To know more about Lithium Carbonate visit:
https://brainly.com/question/15127937
#SPJ1
Why reaction between phosphorus and oxygen produces four atoms of phosphorus and five atoms of oxygen
Answers
Answer:
Reaction between an oxygen and a phosphorus will produce oxides of phosphorus.
Explanation:
Reaction between oxygen and phosphorus produces four atoms of phosphorus with five atoms of oxygen. Sometimes it produces two atoms of phosphorus and five atoms of oxygen and sometimes four atoms of phosphorus and six atoms of oxygen. It does so depending upon the availability of oxygen. The size of phosphorus atom interferes with the ability to form a double bonds to the other elements, such as nitrogen, oxygen, etc.
using the thermodynamic information in the aleks data tab, calculate the boiling point of phosphorus trichloride . round your answer to the nearest degree.
Answers
The boiling point of phosphorus trichloride using the thermodynamic information in the aleks data tab is approximately 77°C.
To calculate the boiling point of phosphorus trichloride using the thermodynamic information in the ALEKS data tab, we need to find the standard enthalpy of vaporization (ΔHvap) and the standard entropy of vaporization (ΔSvap) for the compound.
From the ALEKS data tab, we can find the following thermodynamic information for phosphorus trichloride:
ΔHf°(g) = -284.5 kJ/mol (standard enthalpy of formation of gas phase)
S°(g) = 311.7 J/mol∙K (standard entropy of gas phase)
Using the Clausius-Clapeyron equation:
ln(P2/P1) = (-ΔHvap/R)((1/T2) - (1/T1))
where P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively, and R is the gas constant (8.314 J/mol∙K).
We can rearrange the equation to solve for the boiling point (T2) at a given vapor pressure (P2):
T2 = (-ΔHvap/R)((ln(P2/P1)) + (1/T1))^-1
Assuming a standard pressure of 1 atm (760 torrs), we can use the following data to calculate the boiling point of phosphorus trichloride:
P1 = 1 atm
P2 = 760 torr = 0.997 atm
ΔHvap = ΔHf°(g) + RT
ΔSvap = S°(g)
Substituting the values into the equation, we get:
ΔHvap = (-284.5 kJ/mol) + (8.314 J/mol∙K)(298 K) = -260.6 kJ/mol
T2 = (-ΔHvap/R)((ln(P2/P1)) + (1/T1))^-1
T2 = (-(-260.6 kJ/mol)/(8.314 J/mol∙K))((ln(0.997/1)) + (1/298 K))^-1
T2 = 77°C (rounded to the nearest degree)
Therefore, the boiling point of phosphorus trichloride is approximately 77°C.
Learn more about boiling point at https://brainly.com/question/40140
#SPJ11
PLEASE HELP!! Which object listed best represents the following energy transformation: electrical -> thermal?
a. flashlight
b. electric saw
c. toaster
d. gas powered scooter
e. the sun
f. wind turbine
g. iPod
h. Stereo Speaker Vibrating
Answers
Answer:
The right option is (c) toaster
In which reaction is precipitation occurring? a. mgcl2(aq) cuso4(aq) → cucl2(aq) mgso4(aq) b. cdso4(aq) k2s(aq) → cds(s) k2so4(aq) c. naoh(aq) nh4cl(aq) → nacl(aq) nh4oh(aq) d. k2so4(aq) naoh(aq) → k2oh(aq) naso4(aq) e. hno3(aq) koh(aq) → kno3(aq) h2o(l)
Answers
The reaction in which precipitation reaction occurs is b. CdSo4(aq) +K2S(aq) → CdS(s) +K2SO4(aq).
A precipitation reaction is a double displacement reaction in which a solid salt is formed. If we look at all the given equations then it's only second reaction in which a solid salt (CdS) is formed.
Precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate
The reaction in which precipitation reaction occurs is b. CdSo4(aq) +K2S(aq) → CdS(s) +K2SO4(aq).
Since, the precipitate is formed only in second equation, the correct choice is B.
To learn more about precipitation reaction check the link below:
https://brainly.com/question/20469884
#SPJ4
PLEASE HELP: A cook in a restaurant is using a blender to make soup. The cook plugs the blender into a wall outlet and turns it on. The motor of the blender turns on and moves the blades. The turning blades help blend ingredients for the soup. Which of the following energy transformations describes one of the changes that takes place when the cook uses the blender?(1 point)
Electrical energy is changed into heat energy.
Electrical energy is changed into mechanical energy.
Heat energy is changed into electrical energy.
Mechanical energy is changed into electrical energy
Answers
Electrical energy is changed into mechanical energy which takes place when the cook uses the blender. So, the correct option is B.
What is Electrical energy?Electrical energy is defined as the energy related to the forces on electrically-charged particles and the motion of those particles whose energy is supplied by a combination of current and electric potential that is delivered by a circuit.
Electrical energy is present all around us in many different forms, where some of the best examples of electrical energy are using car battery electrical energy in electrical systems, wall outlet to transfer electrical energy to charge our phones, and our muscles that uses electrical energy to contract and relax. Electrical energy is changed into mechanical energy which takes place when the cook uses the blender.
Therefore, the correct option is B.
Learn more about Electrical energy, here:
https://brainly.com/question/16182853
#SPJ1
What is a decomposition reaction?
Answers
Answer:
The process or effect of simplifying a single chemical entity into two or more fragments.
Explanation:
Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity into two or more fragments. Chemical decomposition is usually regarded and defined as the exact opposite of chemical synthesis.
Answer:
What are some examples of decomposition reactions?
Production of calcium oxide or quicklime.
Production of lithium oxide.
Preparation of oxygen and carbon dioxide.
In metallurgy, for the extraction of metals from their oxides and chlorides through electrolytic decomposition.
Energy Transformation: A home is heated with geothermal energy
(What energy forms are used and how do they go from each form to get to the final form)
Answers
Answer: they convert heat to electricity.
Explanation:
A compound has an empirical formula of
CH20. What is its molecular formula, if its
molar mass is 180 g/mol?
(C=12.01 amu, H=1.008 amu, O=16.00 amu)
Answers
Answer:
C6H12O6
Explanation:
Molar mass of empirical formula:
12.01+2*1.008+16=30.026
Divide molar mass of molecular formula by 30.026
180/30=~6
Scale up the empirical formula by a factor of 6.
C6H12O2 (glucose)
Answer:
C6H12O2
Explanation:
Scale up the empirical formula by a factor of 6.
C6H12O2 (glucose)
12. what mass of glycerin (c3h8o3), a nonelectrolyte, must be dissolved in 200.0 g water to give a solution with a freezing point of -1.50 °c?
Answers
The mass of the glycerin, C₃H₈O₃ a nonelectrolyte, that must be dissolved in 200.0 g water to give a solution with the freezing point of -1.50 °C is 14.8 g.
Mass of solvent = 200.0 g
Depression at freezing point = - 1.50 °C
Kf = 1.86 °C / m
The molality is given as :
0 - ΔT = Kf × m × i
Where ,
ΔT = - 1.50 °C
Kf = 1.86 °C / m
m = molality = ?
i = 1
m = 1.50 / 1.86
m = 0.806 m
The molality = ( mass of solute × 1000) / ( molar mass of solute × mass of solvent)
Mass of solute =( 0.806 × 92 × 200 ) / 1000
Mass of the solute = 14.8 g
To learn more about freezing point here
https://brainly.com/question/13122709
#SPJ4
Why is cerium sulfate (Ce2(SO4)3) the only compound that experiences a dip in solubility as temperature increases?
Answers
Answer: Because its dissolution is exothermic
Explanation:
What is the percent mass of 55.0g NH4Cl dissolved in 137g water?
Answers
Step 1
% by mass:
Mass of solute ---- 100 of solution
Mass of solution = mass of solute + mass of solvent = 55.0 g + 137 g = 192 g
Solute = NH4Cl
Solvent = Water (H2O)
-----------------------------
Step 2
Procedure:
55.0 g NH4Cl --------- 192 g solution
X ---------- 100 g solution
X = 29 g NH4Cl = 29 % by mass approx.
Answer: 29 % by mass
Which of the following represents an endothermic reaction?
Select one:
a. S(s) +O2(g) → SO2(g) ΔH = -297kJ
b. 2NO2(g) → N2(g) + 2O2(g) + 33.8kJ
c. N2(g) + 2O2(g) + 90.4kJ → 2NO2
d. N2H4(g) + O2(g) → N2(g) +2H20(g) + 627.6 kJ
Answers
B because decomposition reactions are often caused by the application of heat to break up the bonds within the molecules, which will form the molecules that made up the decomposed molecule.
Rank the following molecules in terms of their carbonyl stretching frequency, v(C=O), in the infrared spectrum. 2-cyclohexenone 2,4-cyclohexadienone cyclohexanone Highest Frequency Carbonyl Stretch Lowest Frequency Carbonyl Stretch 2.4-cyclohexaceenone cyclohexenone 2-cyclohexenone
Answers
The carbonyl stretching frequency in the infrared spectrum depends on the nature of the carbonyl group and the adjacent functional groups or substituents. Based on this, we can rank the given molecules in terms of their carbonyl stretching frequency, from highest to lowest:
2,4-cyclohexadienone > 2-cyclohexenone > cyclohexenone > cyclohexanone
In general, a carbonyl group adjacent to an electron-withdrawing group will have a higher stretching frequency compared to a carbonyl group adjacent to an electron-donating group.
In 2,4-cyclohexadienone, the two carbonyl groups are conjugated with each other and with the double bonds in the ring, resulting in a very high carbonyl stretching frequency. In 2-cyclohexenone, the carbonyl group is conjugated with the double bond in the ring, resulting in a slightly lower stretching frequency.
In cyclohexenone, the carbonyl group is adjacent to a single double bond in the ring, resulting in a lower stretching frequency compared to 2-cyclohexenone. In cyclohexanone, the carbonyl group is not conjugated with any other functional group, resulting in the lowest carbonyl stretching frequency among the given molecules.
For more question on infrared spectrum click on
https://brainly.com/question/5951360
#SPJ11
BRAINLIEST PLEASEEE HELPLP 5. In a lab experiment, 2.5 grams of sodium bicarbonate is heated and decomposed into
sodium carbonate, carbon dioxide, and water vapor when heated. The actual yield of
sodium carbonate produced in the experiment is 2.04 grams. The theoretical yield of
each product is recorded in the data table below.
Using this data, determine the percent yield for sodium carbonate?
(Round Your Answer to the Nearest Whole Number)

Answers
Answer:
Explanation:
Sodium bicarbonate,
NaHCO
3
, will decompose to form sodium carbonate,
Na
2
CO
3
, water, and carbon dioxide,
CO
2
2
NaHCO
3(s]
→
Na
2
CO
3(s]
+
CO
2(g]
+
H
2
O
(g]
Notice that you have a
2
:
1
mole ratio between sodium bicarbonate and sodium carbonate. This means that the reaction will produce half as many moles of the latter than whatever number of moles of the former underwent decomposition.
Use sodium carbonate's molar amss to determine how many moles you'd get in that sample
0.685
g
⋅
1 mole NaHCO
3
84.007
g
=
0.008154 moles NaHCO
3
Now, if the reaction were to have a
100
%
yield, it would produce
0.008154
moles NaHCO
3
⋅
1 mole Na
2
CO
3
2
moles NaHCO
3
=
0.004077 moles Na
2
CO
3
Use the molar mass of sodium carbonate to determine how many grams would contain this many moles
0.004077
moles
⋅
105.99 g
1
mole
=
0.4321 g Na
2
CO
3Sodium bicarbonate,
NaHCO
3
, will decompose to form sodium carbonate,
Na
2
CO
3
, water, and carbon dioxide,
CO
2
2
NaHCO
3(s]
→
Na
2
CO
3(s]
+
CO
2(g]
+
H
2
O
(g]
Notice that you have a
2
:
1
mole ratio between sodium bicarbonate and sodium carbonate. This means that the reaction will produce half as many moles of the latter than whatever number of moles of the former underwent decomposition.
Use sodium carbonate's molar amss to determine how many moles you'd get in that sample
0.685
g
⋅
1 mole NaHCO
3
84.007
g
=
0.008154 moles NaHCO
3
Now, if the reaction were to have a
100
%
yield, it would produce
0.008154
moles NaHCO
3
⋅
1 mole Na
2
CO
3
2
moles NaHCO
3
=
0.004077 moles Na
2
CO
3
Use the molar mass of sodium carbonate to determine how many grams would contain this many moles
0.004077
moles
⋅
105.99 g
1
mole
=
0.4321 g Na
2
CO
3
By using the given data, the percent yield for sodium carbonate (Na₂CO₃) is equal to 127.
How to calculate percent yield?Percent yield of any data can be calculated as:
% yield = (Actual value / Theoretical value) × 100
In the question actual yield of sodium carbonate is given, which is equal to 2.04 grams. And in the table theoretical yield of sodium carbonate also given, which is equal to 1.60 grams.
Now putting these value in the above equation, we get:
% yield = (2.04 / 1.60) × 100 = 127
Hence, percent yield of sodium carbonate (Na₂CO₃) is 127.
To learn more about percent yield, visit the below link:
https://brainly.com/question/11963853
The ground-state electron configuration for an element contains an unpaired 3s electron.
Answers
Answer:
Na
Sodium is located in the third row of the periodic table, where the 3s3s3, s orbital is being filled. Because sodium is the first of the two 3s3s3, s elements, its ground-state electron configuration contains a single, unpaired 3s3s3, s electron.
Explanation:
its late but ik someone can use it
The ground-state electron configuration for an element contains an unpaired 3s electron is Sodium, Na.
The ground state electron configuration of an element shows a series of how its electrons are arranged in thier orbitals at their lowest energy state.
The element which contained an unpaired 3s electron is the Alkali metal, Sodium.
Sodium, Na has atomic number of 11 and at ground state and will therefore be written as 1s²2s²2p⁶3s¹ We can also write the condensed form since the core electrons is the same with Ne, a noble gas which is [Ne]3s1.
The s orbital should contain at most 2 electrons , since for sodium, it has 1 electron in the 3s orbital, we say it is unpaired.
See more here:https://brainly.com/question/2071096
how will the types of bonds being broken.formed leading to the two different tpyes of products affect the overall energy of the reactions g
Answers
The types of bonds being broken and formed will impact the overall energy of the reaction, and this can be determined by examining whether the reaction is endothermic or exothermic.
The type of bonds being broken and formed in a reaction will have a significant impact on the overall energy of the reaction. When strong bonds are broken, more energy is required as compared to breaking weaker bonds.
Similarly, when strong bonds are formed, more energy is released as compared to forming weaker bonds. If the reaction involves breaking strong bonds and forming weak bonds, it will be an endothermic reaction, meaning that it requires energy to occur.
Conversely, if the reaction involves breaking weak bonds and forming strong bonds, it will be an exothermic reaction, meaning that it releases energy.
To learn more about : energy
https://brainly.com/question/30083274
#SPJ11
Which of the following best illustrates the law of corkervation of energy?
A
The work done stretching a spring is transformed into stored energy in the spring
B
Leak-proof batteries are able to store chemical energy for long periods of time.
С
Ajogger conserves her energy by reducing the rate at which she jogs.
D
Ice water changes into liquid water when it absorbs heat energy.
Answers
Answer:
A . The work done stretching a spring is transformed into stored energy in the spring
Explanation:
The work done stretching a spring is transformed into stored energy in the spring is clear indication of the law of conservation of energy.
The law of conservation of energy states that "energy is neither created nor destroyed but transformed from one form to another".
In this problem, mechanical energy is used to stretch the spring. The mechanical energy is converted to stored potential energy.This is an energy transformation process.