The mass number of an atom is equal to the total number of protons and neutrons.
is it true or false
Answers
Related Questions
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 grams of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C to 32.33 ∘C.
Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in a separate experiment, is 5.11kJ/∘C.
Express the change in energy in kilojoules per mole to three significant figures.
Can you explain each step, please?
Answers
ΔErxn for the combustion of naphthalene is approximately 5150 kJ/mol to three significant figures.
To find ΔErxn for the combustion of naphthalene, follow these steps:
1. Calculate the change in temperature (ΔT):
ΔT = Final Temperature - Initial Temperature
ΔT = 32.33°C - 24.25°C = 8.08°C
2. Calculate the heat absorbed by the calorimeter (q_cal):
q_cal = Heat Capacity × ΔT
q_cal = 5.11 kJ/°C × 8.08°C = 41.249 kJ
3. Find the moles of naphthalene (n):
n = mass of naphthalene / molar mass of naphthalene
Molar mass of naphthalene (C10H8) = (10 × 12.01 g/mol) + (8 × 1.008 g/mol) = 128.17 g/mol
n = 1.025 g / 128.17 g/mol ≈ 0.008 mol
4. Calculate ΔErxn per mole:
ΔErxn = q_cal / n
ΔErxn = 41.249 kJ / 0.008 mol ≈ 5150 kJ/mol
Therefore, ΔErxn for the combustion of naphthalene is approximately 5150 kJ/mol to three significant figures.
To learn more about combustion, refer below:
https://brainly.com/question/15117038
#SPJ11
What's the meaning of love?
Answers
Answer: Love is when you feel like you can touch the sky when your around that person, when your at your best self or that person and when you put their needs before yours because your needs just don't matter. When you feel like you can't stop thinking about that person or you can stop smiling when you think of them. Its when you feel this warm, safe feeling when your around them. And if you really love them then my answer should be true for you.
Explanation:
because iv'e felt and have been in love before....
Answer:
Love is an intense, deep affection for another person. Love also means to feel this intense affection for someone. Love can also refer to a strong like for something or to like something a lot. Love has many other senses both as a verb and a noun
How does covalent bonding take place?
A. A nonmetallic element like Fluorine is attracted to a metallic element like
Sodium.
B. A metallic element like Sodium transfers an electron to a non-metallic element
like Fluorine.
C. Two or more non-metallic elements share electrons to attain stability.
D. Two or more non-metallic elements of the same kind form strong forces of
attraction.
Answers
What is the oxidation number of zinc in zncl2?
Answers
The oxidation number of the zinc in the ZnCl₂ is the +2.
The oxidation number, is also called as the oxidation state, the total number of the electrons that an atom either will gains or will loses in order to form the chemical bond with the another atom.
The oxidation number of the chlorine = -1
The oxidation number of the Zn is as follows :
Zn + 2( -1 ) = 0
Zn - 2 = 0
Zn = + 2.
The Oxidation number of an atom can be defined as the charge that the atom appears will have on forming the ionic bonds with the other heteroatoms.
To learn more about oxidation number here
https://brainly.com/question/29257381
#SPJ4
how are atoms structured
Answers
Answer:
they got protons, electrons, and neutrons
Explanation:
Answer:
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
Explanation:
1. Take 2-3 crystals of potassium permanganate and dissolve them in 100 mL of water.
2. Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
3. Take out 10 mL of this solution and put it into another 90 mL of clear water.
4. Keep diluting the solution like this 5 to 8 times.
5. Is the water still coloured?
Answers
Answer:
2
Explanation:
1. Take 2-3 crystals of potassium permanganate and dissolve them in 100 mL of water.
2. Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
3. Take out 10 mL of this solution and put it into another 90 mL of clear water.
4. Keep diluting the solution like this 5 to 8 times.
5. Is the water still coloured?
The rate of a certain reaction is given by the following rate law:
rate = k [H2]2[NH3]
Use this information to answer the questions below.
1. What is the reaction order in H2? ____________
2. What is the reaction order in NH3 ? _____________
3. What is overall reaction order? __________
4. At a certain concentration of H2 and NH3 the initial rate of reaction is 0.740 M / s. What would the initial rate of the reaction be if the concentration of H2 were doubled? Round your answer to 3 significant digits. ________________M/s
5. The rate of the reaction is measured to be 6.0 x 104 M / s when [H2] = 0.98 M and [NH3] = 0.31 M. Calculate the value of the rate constant. Round your answer to 2 significant digits. k = ______________ M-2 x s-1
Answers
Explanation :
1. The reaction order in H2 is 2.
2. The reaction order in NH3 is 1.
3. The overall reaction order is 3.
4. The initial rate of reaction would be 2.96 M/s.
5. The value of the rate constant is 6.1 x 105
M-2 x s-1.
Step-by-step explanation:
1. The rate law is given by rate = k [H2]2 [NH3].
The power with which H2 is raised is 2.
Therefore, the reaction order in H2 is 2. 2.
The rate law is given by rate = k [H2]2 [NH3].
The force with which NH3 is raised is 1.
Therefore, the reaction order in NH3 is 1.3.
The sum of the reaction orders in H2 and NH3 is 2 + 1 = 3.
Therefore, the overall reaction order is 3.4.
The initial rate of reaction is given as 0.740 m/s.
If the concentration of H2 is doubled, then its concentration becomes [H2].
The new rate can be calculated using the equation:
rate1/rate2 = (k [H2]12 [NH3])/(k [H2]22 [NH3]. 0.740/rate2 = ([H2]/2) 2/[H2]20.740/rate2 = (1/2) 2rate2 = 2.96 M/s.
5.
The rate constant (k) can be calculated using the equation: rate = k [H2]2 [NH3]. k = rate/([H2]2[NH3]) k = 6.0 x 104/(0.98)2(0.31)k = 6.1 x 105 M-2 x s1
(rounded to 2 significant digits).
To know more about the order of the reaction https://brainly.com/question/1769080
#SPJ11
Determine the mass of nitrogen that is produced when 7.80 grams of dimitrogen tetrahydride reacts with hydrogen peroxide (H202). NaH. + 2H202 + N2 + 4H20
Answers
4.33 grams of nitrogen are produced when 7.80 grams of dinitrogen tetrahydride reacts with hydrogen peroxide.
To determine the mass of nitrogen (N2) produced when 7.80 grams of dinitrogen tetrahydride (NaH) reacts with hydrogen peroxide (H2O2), we need to calculate the stoichiometry of the balanced chemical equation and use the molar masses of the compounds involved.
The balanced chemical equation is:
2NaH + 2H2O2 → N2 + 4H2O
From the equation, we can see that 2 moles of NaH react with 2 moles of H2O2 to produce 1 mole of N2. To find the molar mass of N2, we add the atomic masses of two nitrogen atoms:
Molar mass of N2 = 2 × Atomic mass of nitrogen = 2 × 14.01 g/mol = 28.02 g/mol
Now, let's calculate the number of moles of NaH:
Moles of NaH = Mass of NaH / Molar mass of NaH
Moles of NaH = 7.80 g / (22.99 g/mol + 1.01 g/mol) ≈ 0.3088 mol
According to the balanced equation, the molar ratio of NaH to N2 is 2:1. Therefore, the moles of N2 produced will be half the moles of NaH used:
Moles of N2 = 0.3088 mol / 2 ≈ 0.1544 mol
Finally, to find the mass of nitrogen produced, we multiply the moles of N2 by the molar mass of N2:
Mass of N2 = Moles of N2 × Molar mass of N2
Mass of N2 = 0.1544 mol × 28.02 g/mol ≈ 4.33 g
Therefore, approximately 4.33 grams of nitrogen are produced when 7.80 grams of dinitrogen tetrahydride reacts with hydrogen peroxide.
For more question on nitrogen visit;
https://brainly.com/question/1380063
#SPJ8
Balance the following equation in acidic solution using thelowest possible integers and give the coefficient of water.NH4+(aq) +Cr2O72-(aq) ?Cr3+(aq) + N2(g)a.9b.1c.7d.3
Answers
The coefficient of water in the balanced equation is 7. Option C is correct.
Balanced chemical equation for the given reaction in acidic solution is;
NH₄⁺(aq) + Cr₂O₇²⁻(aq) + 14H⁺(aq) → Cr³⁺(aq) + N₂(g) + 12H₂O(l)
To balance the equation, we start by balancing the oxygen atoms by adding water molecules to the appropriate side;
NH₄⁺(aq) + Cr₂O₇²⁻(aq) + 14H⁺(aq) → Cr³⁺(aq) + N₂(g) + 7H₂O(l)
Now, we balance the hydrogen atoms by adding H+ ions to the appropriate side;
NH₄⁺(aq) + Cr₂O₇²⁻(aq) + 14H⁺(aq) → Cr³⁺(aq) + N₂(g) + 7H₂O(l)
Finally, we balance the charge by adding electrons to the appropriate side;
NH₄⁺(aq) + Cr₂O₇²⁻(aq) + 14H⁺(aq) → Cr³⁺(aq) + N₂(g) + 7H₂O(l) + 14e⁻
Therefore, the coefficient of water is 7.
Hence, C. is the correct option.
To know more about coefficient here
https://brainly.com/question/22241464
#SPJ4
In one to two sentences, explain a similarity and a difference between the particles in liquid water at 100°C and the particles in
steam at 100°C. (2 points)
Answers
A similarity between the particles in liquid water and the particles in steam is their chemical composition whereas a difference between the particles in liquid water and the particles in steam is their state of matter.
What are liquid and steam?Steam refers to the gas which is formed when water passes from the state of liquid to the gaseous state. Steam is formed when water molecules break the bonds i.e. hydrogen bonds that keep them together while on the other hand, a liquid is a sample of matter that takes the shape of a container in which it is placed. The term liquid is used to show the state or condition of matter.
So we can conclude that chemical composition is the similarity between liquid and steam while on the other hand, the difference in the state of matter.
Learn more about water here: https://brainly.com/question/1313076
#SPJ1
How many grams of Potassium are in 10 moles of potassium?
Answers
Answer:
You can view more details on each measurement unit: molecular weight of Potassium or grams The molecular formula for Potassium is K. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Potassium, or 39.0983 grams.
Explanation:
The form of energy from the sun can be transformed into the energy needed for a tree to grow. What types of energy occur in this transformation?
Question 5 options:
Electromagnetic - mechanical
Potential - kinetic
Light - chemical
Nuclear - thermal
Answers
The form of energy from the sun can be transformed into the energy needed for a tree to grow is light to chemical energy.
What is energy transformation?Energy transformation is a process in which one form of energy is transfered into another form without any loss of energy.
Sun provides solar energy to the earth surface and that will be used by plants and trees for the production of food to grow. So that plants will convert the solar energy means light energy into chemical energy for their use.
Hence conversion of light energy to chemical energy takes place.
To know more about energy transformation, visit the below link:
https://brainly.com/question/961052
#SPJ1
Car and Motorcycle engines are controlled by what chemical reaction?
Answers
Car and Motorcycle engines are controlled by a chemical reaction which is controlled by gasoline and oxygen.
What is a Chemical reaction?A chemical reaction may be defined as a process that significantly involves the chemical transformation of one set of chemical substances to another. This process involves the utilization of substances known as reactants that are transformed into other substances known as products.
The fuel in cars and motorcycles delivers the source of energy to them in order to move from one place to another. This fuel significantly contains gasoline which reacts with atmospheric oxygen and results in a small explosion which derives some sort of energy that is finally consumed by the vehicle's engine.
Apart from this, the combustion of fuel is also facilitated in the presence of oxygen which liberates extremely high amounts of carbon dioxide in the atmosphere. This reaction has also occurred in the engines of vehicles.
Therefore, car and Motorcycle engines are controlled by a chemical reaction which is controlled by gasoline and oxygen.
To learn more about Chemical reactions, refer to the link:
https://brainly.com/question/11231920
#SPJ2
what does Molecular mean in a simple answer?
Answers
i nees help plzzz.
this is a science question
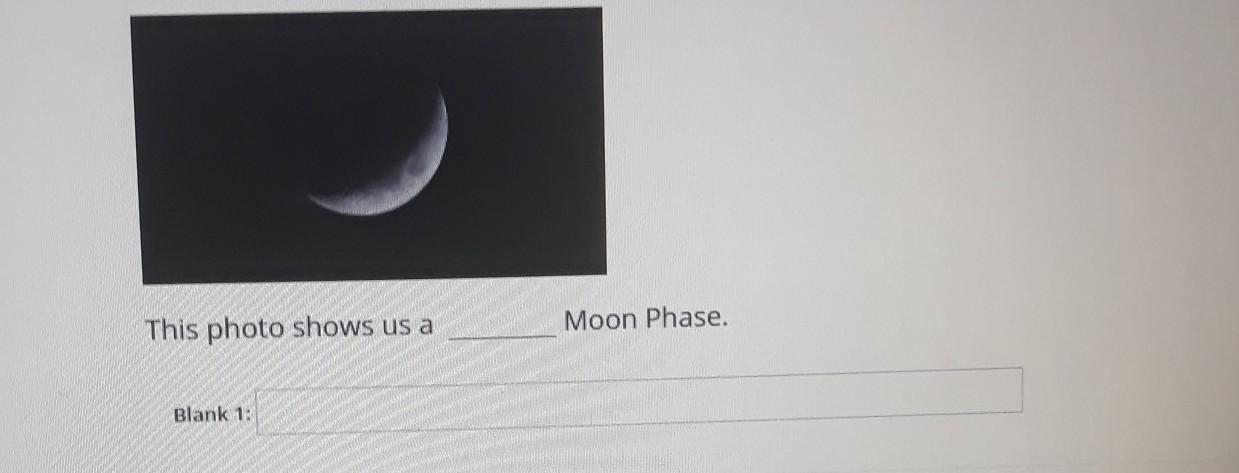
Answers
A volume of 1.50 L of argon gas is confined in a cylinder with a movable piston under a constant pressure of 1.22 x 10^5 Pa. when 1.25 kJ of energy in the form of heat is transferred from the surrounding to the gas, the internal energy of the gas increases by 1.11 kJ. What is the final volume of Argon gas in the cylinder?
Answers
The final volume of Argon gas in the cylinder is 1.50115 L using the first law of thermodynamics.
To solve this problem, we need to use the first law of thermodynamics which states that the change in internal energy of a system is equal to the heat transferred to the system minus the work done by the system.
In this case, we know that the internal energy of the gas increased by 1.11 kJ when 1.25 kJ of energy in the form of heat was transferred to the gas. Therefore, the work done by the system must be equal to the difference between these two values:
Work = Heat - Change in Internal Energy (W = Q - ΔU)
Work = 1.25 kJ - 1.11 kJ
Work = 0.14 kJ
Now, we can use the formula for work done by a gas:
Work = Pressure(P) x Change in Volume (ΔV)
We know that the pressure is constant at 1.22 x 10⁵ Pa and we need to find the change in volume. Rearranging the formula, we get:
Change in Volume = Work / Pressure
Change in Volume = 0.14 kJ / 1.22 x 10⁵ Pa
Change in Volume = 1.15 x 10⁻⁶ m³
Finally, we can calculate the final volume of the gas by adding the change in volume to the initial volume:
Final Volume = Initial Volume + Change in Volume
Final Volume = 1.50 L + 1.15 x 10⁻⁶ m³
Note that we need to convert the change in volume from cubic meters to liters:
Final Volume = 1.50 L + 0.00115 L
Final Volume = 1.50115 L
Therefore, the final volume of the argon gas in the cylinder is 1.50115 L.
To know more about the first law of thermodynamics, refer here:
https://brainly.com/question/3808473#
#SPJ11
There are 12 liters of nitrogen gas in a sample. How many moles of N2 in this sample?
Answers
Answer:
0.54 moles of N2
Explanation:
First remember to find the volume in moles, 1 mole equals 22.4 liters.
So now use the dimensional analysis to show your work.
12 liters of N2 * 1 mol /22.4 liters of N2
Now calculate this. 12 * 1/22.4 or 12/ 22.4
12/22.4 = 0.535714286.
12 has two significant digits.
With that, The answer rounds to 0.54.
So that the final answer is 0.54 moles.
Hope it helped!
How do the ideas of electrolytes and IV fluids relate?
Answers
Answer:
Electrolytes, particularly sodium, help the body maintain normal fluid levels in the fluid compartments because the amount of fluid a compartment contains depends on the amount (concentration) of electrolytes in it. If the electrolyte concentration is high, fluid moves into that compartment (a process called osmosis).
Explanation:
Answer:
Electrolytes are minerals in your body that have an electric charge. They are in your blood, urine, tissues, and other body fluids. Electrolytes are important because they help
Balance the amount of water in your body
Balance your body's acid/base (pH) level
Move nutrients into your cells
Move wastes out of your cells
Make sure that your nerves, muscles, the heart, and the brain work the way they should
Sodium, calcium, potassium, chloride, phosphate, and magnesium are all electrolytes. You get them from the foods you eat and the fluids you drink.
The levels of electrolytes in your body can become too low or too high. This can happen when the amount of water in your body changes. The amount of water that you take in should equal the amount you lose. If something upsets this balance, you may have too little water (dehydration) or too much water (overhydration). Some medicines, vomiting, diarrhea, sweating, and liver or kidney problems can all upset your water balance.
Treatment helps you to manage the imbalance. It also involves identifying and treating what caused the imbalance.
hope it's help you plz mark as brain listPlease! I need help
Describe how water molecules can hydrate various substances.
Answers
Answer:
The two hydrogen atoms and one oxygen atom within water molecules (H2O) form polar covalent bonds. ... As a result of water's polarity, each water molecule attracts other water molecules because of the opposite charges between them, forming hydrogen bonds.
Explanation:
Not my work, but I hope this helps!
For which of the following reactions will a decrease in pressure shift the equilibrium to the left?
A). 2A2 (g) + B2 (g) --> 2A2B (g)
B). 2AB (g) --> A2 (g)+ B2 (g)
C). 2A2F3 (g) --> 4A (g) + 3F2 (g)
D). 2B (s) + 2HA (aq) --> 2BA (aq) + H2 (g)
Answers
Answer: I just took the test, the answer is A.
Explanation:
What is the name of this molecular compound ? F6I5 PLEASE AND THANK YOU
Answers
Answer:
Hexaflourine Pentaiodide?
Explanation:
f = flourine (6 = hexa)
i = iodine (5 = penta) + ide
Will the reaction 2 NO2 -> N2O4 occur at 25C?Delta H = - 57.2KJDelta S= - 0.176 KJ/K
Answers
Step 1
At constant temperature and pressure, the change in Gibbs free energy is defined as ΔG = ΔH − T ΔS
------------
Step 2
When ΔG < 0 (negative) the reaction will proceed spontaneously
------------
Step 3
Information provided:
Delta H = ΔH = - 57.2KJ
Delta S = ΔS = - 0.176 KJ/K
T = 25 °C + 273 = 298 K
-----------
Step 4
Procedue:
ΔG = ΔH − T ΔS = -57.2 kJ - (-0.176 kJ/K) x 298 K = - 4.752 kJ
Therefore, the reaction will occur spontaneously.
Answer: Yes, it wil (because of the procedure)
name some animals and plants that live on land?
please mark as brainliest
Answers
Answer:
cats, ants, spiders, elephants, porcupine, tiger, kangaroo, and ostrich. Mosses, hornworts, liverworts, lycophyte, seed plants, pteridophytes, and archaeopteridales.
Explanation:
Allyson and Cami add 25 mL of 3 M hydrochloric acid (HCI) to
beakers containing 0 mL, 25 mL, 50 mL, and 75 mL of distilled
water. The students then drop 5 cm of magnesium (Mg) ribbon into each beaker and measure the time for the magnesium to completely react with the acid. The results are shown in the data table.
Which statement best explains the change in reaction time as the amount of water increases?
Select one:
- The magnesium remains separated from the acid.
- The volume of the mixture increases.
- The water is cooled by the acid.
- The concentration of the acid decreases.

Answers
The statement that best explains the change in reaction time as the amount of water increases is that the concentration of the acid decreases. Option 4.
Rate of reaction and concentrationThe rate of chemical reactions increases with an increase in the concentration of the reactants, all other things being equal.
When hydrochloric acid is diluted with water, its concentration decreases. A lower concentration of acid means that there are fewer acid particles available to react with the magnesium, so the reaction time increases.
This is consistent with the data table, where the reaction time increases as the amount of water increases.
Therefore, the correct answer is that the concentration of the acid decreases.
More on concentration and reaction rates can be found here: https://brainly.com/question/13764840
#SPJ1
Which of he following genetic descriptions are at the molecular, cellular, organismal or population level?
1. A person's blood cells be blood type A, B, or O.
2. The enzyme in type A people adds a sugar group to the blood cell membrane.
3. B blood type is most prevalent in people from Central Asia.
4. A rabbit carrying two copies of the Himalayan coat color allele has black paws.
5. The enzyme for black pigment functions only at temperatures below 20 degrees C.
6. One percent of all rabbits carry a Himalayan coat color allele.
Answers
Molecular level: A person's blood cells having blood type A, B, or O is a genetic description at the molecular level. It involves the presence or absence of specific alleles that determine the blood type.
Molecular level: The presence of an enzyme in type A individuals that adds a sugar group to the blood cell membrane is a molecular-level genetic description. It relates to the specific function of an enzyme determined by genetic variation. Population level: The prevalence of blood type B in people from Central Asia is a genetic description at the population level. It refers to the frequency distribution of a particular blood type within a specific geographic region. Cellular level: A rabbit carrying two copies of the Himalayan coat color allele having black paws is a genetic description at the cellular level. It involves the expression and manifestation of a specific coat color allele in the cells of the rabbit. Molecular level: The temperature-dependent functionality of the enzyme responsible for black pigment in rabbits is a genetic description at the molecular level. It indicates the specific conditions under which the enzyme can effectively carry out its function. Population level: The statement that one percent of all rabbits carry a Himalayan coat color allele is a genetic description at the population level. It represents the frequency of a particular allele within a rabbit population.
learn more about Molecular here:
https://brainly.com/question/156574
#SPJ11
in 1963, what did scientists officially name the element at 101 on the periodic table?
Answers
Answer: The element at 101 on the periodic table was officially named "Mendelevium" in 1963 by a team of scientists at the Lawrence Berkeley National Laboratory in California. The name was chosen to honor the Russian chemist Dmitri Mendeleev, who is credited with the development of the modern periodic table of elements. Mendelevium is a synthetic element that is produced by bombarding lighter elements with charged particles in a particle accelerator. It is a highly unstable element and has no known commercial or industrial uses.
Mendelevium (Md) is a synthetic, radioactive element that was first synthesized in 1955 by a team of scientists at the University of California, Berkeley. It is named after the Russian chemist Dmitri Mendeleev, who is credited with developing the periodic table of elements.
Mendelevium does not occur naturally on Earth and can only be produced through artificial means. It is produced by bombarding lighter elements, such as americium or plutonium, with high-energy particles in a particle accelerator. The resulting atoms of mendelevium are highly unstable and decay quickly, with a half-life of only a few hours.
Because of its short half-life and the difficulty in producing it, mendelevium has no commercial or industrial uses. However, it is important for scientific research and has been used in studies of nuclear physics and chemistry. In addition, the study of mendelevium and other synthetic elements can help scientists better understand the fundamental properties of matter and the structure of the periodic table.
In 1963, scientists officially named the element at position 101 on the periodic table as "Mendelevium."
Mendelevium is a synthetic element, meaning it is not found naturally on Earth but can be created through nuclear reactions. It is produced through the bombardment of lighter elements with high-energy particles. The element was named in honor of Dmitri Mendeleev, the Russian chemist who developed the periodic table as we know it today. Mendelevium has the symbol "Md" and is part of the actinide series of elements, which have atomic numbers ranging from 89 to 103.
Due to its high atomic number, Mendelevium is a heavy and radioactive element with a short half-life, making it challenging to study and find practical applications for it. The discovery of Mendelevium was a significant achievement in the field of nuclear science, as it opened up new possibilities for studying the properties of heavy elements and their isotopes.
To learn more about periodic table visit:
https://brainly.com/question/1173237
#SPJ11
Complete the sentences to explain your choice. Match the words in the left column to the appropriate blanks in the sentences on the right. When comparing HF and HCl, HCl is a stronger acid because the bond is ___When comparing H2O and HF, HF is a stronger acid because the bond is ___When comparing H2Se and H2S, H2Se is a stronger acid because the bond is ___less polar more polarweakerstronger
Answers
Answer:
HCl is a stronger acid because the bond is weaker
Hf is a stronger acid because the bond is more polar
H2Se is a stronger acid because the bond is weaker.
Explanation:
Weaker and more polar acids make stronger acids.
a. When comparing HF and HCl, HCl is a stronger acid because the bond is stronger.
b. When comparing H2O and HF, HF is a stronger acid because the bond is weaker.
c. When comparing H2Se and H2S, H2Se is a stronger acid because the bond is weaker.
In comparing the acidity of acids, we can consider the strength of the bond between the hydrogen atom and the non-metallic atom in the acid. The weaker the bond, the more easily the hydrogen ion can be donated to a base, making the acid stronger.
a. This is because HCl has a weaker bond between hydrogen and chlorine, and it readily donates hydrogen ions to a base.
b. This is because HF has a stronger bond between hydrogen and fluorine, which makes it harder to donate hydrogen ions to a base.
c. This is because H2Se has a weaker bond between hydrogen and selenium, and it readily donates hydrogen ions to a base.
To know more about "Acids" refer here:
https://brainly.com/question/12814523#
#SPJ11
A compound was found to contain 90.6% lead (Pb) and 9.4% oxygen. What is the empirical formula for this compound?
Answers
Answer:
the answer is 47.9 and ik because I just had that question
The empirical formula of the compound is O₄Pb₃.
What is the empirical formula?
An Empirical system is the chemical system of a compound that offers the proportions of the elements gifted within the compound however not the real numbers or arrangement of atoms. This would be the lowest complete variety ratio of the elements within the compound.
Amount of lead (Pb) = 90.6%
⇒and amount of oxygen = 9.4%
taking the whole number ratio
o = 4
Pb = 3
∴ ⇒O: Pb=4:3
O4Pb3 answer.
Learn more about empirical formula here:-https://brainly.com/question/1603500
#SPJ2
Who is known as “laughing philosopher “ ?
Answers
Answer:
Democritus
Explanation:
How many elements are in C8H3NaBr12?
Answers
Answer:
I don’t get it you have to go more into detail lol then I’ll answer
Explanation: