The partial molar volumes of acetone (propanone) and chloroform (trichloromethane) in a mixture in which the mole fraction of CHCl3 is 0.4693 are 74.166 cm^3mol^-1 and 80.235 cm^3, respectively. What is the volume of a solution of mass 1.000Kg?
Answers
The volume of the solution of mass 1.000Kg in a mixture in which the partial molar volumes of acetone (propanone) and chloroform (trichloromethane) are 74.166 cm^3mol^-1 and 80.235 cm^3 and mole fraction of CHCl3 is 0.4693 is 0.993 L.
We can find the volume of the solution of mass 1.000Kg as follows:
First, we need to calculate the number of moles of each component present in the solution.
since we know,
mole fraction of acetone + mole fraction of chloroform = 1
and, mole fraction of chloroform = 0.4693
So, mole fraction of acetone = 1 - 0.4693
mole fraction of acetone = 0.5307
moles of acetone = 0.5307 × (mass of solution/molar mass of acetone)
moles of acetone = 0.5307 × (1000 g / 58.08 g/mol)
moles of acetone = 9.137 mol
Number of moles of chloroform:
moles of chloroform = 0.4693 × (mass of solution/molar mass of chloroform)
moles of chloroform = 0.4693 × (1000 g / 119.37 g/mol)
moles of chloroform = 3.931 mol
Now, we can use the partial molar volumes to calculate the volume of the solution:
Vsolution = (nCHCl3 × VCHCl3) + (nacetone × Vacetone)
Vsolution = (3.931 mol × 80.235 cm³mol⁻¹) + (9.137 mol × 74.166 cm³mol⁻¹)
Vsolution = 315.403 cm³ + 677.654 cm³
Vsolution = 993.057 cm³ = 0.993 L
Therefore, the volume of the solution of mass 1.000Kg is approximately 0.993 L
Know more about mole fraction here:
https://brainly.com/question/31285244
#SPJ11
The volume of a solution with a mass of 1.000 kg is approximately 691.02 cm^3.
To calculate the volume of the solution, we need to find the moles of acetone and chloroform in the mixture and then use their partial molar volumes.
1. Calculate moles of acetone and chloroform using their mole fractions:
Mole fraction of acetone (C3H6O) = 1 - 0.4693 = 0.5307
Molar mass of acetone = 58.08 g/mol, chloroform = 119.38 g/mol
Mass of the solution = 1.000 kg = 1000 g
Let x be the mass of acetone and y be the mass of chloroform. Then,
x/58.08 = 0.5307, and y/119.38 = 0.4693
x + y = 1000 (since total mass is 1 kg)
2. Solve these equations to find the masses of acetone and chloroform:
From x/58.08 = 0.5307, we get x = 30.82 g
From y = 1000 - x, we get y = 969.18 g
3. Calculate the moles of acetone and chloroform:
Moles of acetone = 30.82 g / 58.08 g/mol = 0.5307 mol
Moles of chloroform = 969.18 g / 119.38 g/mol = 8.121 mol
4. Use the partial molar volumes to find the total volume of the solution:
Volume of acetone = 0.5307 mol × 74.166 cm^3/mol = 39.37 cm^3
Volume of chloroform = 8.121 mol × 80.235 cm^3/mol = 651.65 cm^3
Total volume = Volume of acetone + Volume of chloroform
Total volume = 39.37 cm^3 + 651.65 cm^3 = 691.02 cm^3
So, the volume of a solution with a mass of 1.000 kg is approximately 691.02 cm^3.
To learn more about acetone, refer below:
https://brainly.com/question/13334667
#SPJ11
Related Questions
1. You have 4 moles of NaCl. How many particles are present?
Answers
To calculate the molecules present in the given moles we will apply for Avogadro's number. Avogadro's number tells us that in one mole of any substance there are 6.022x10^23 molecules.
Therefore if we have 4 moles of NaCl we will have:
\(\begin{gathered} moleculesNaCl=GivenmolNaCl\times\frac{6.022\cdot10^{23}moleculesNaCl}{1molNaCl} \\ moleculesNaCl=4molNaCl\times\frac{6.022\cdot10^{23}moleculesNaCl}{1molNaCl} \\ moleculesNaCl=2\times10^{24}moleculesNaCl \end{gathered}\)In 4 moles of NaCl there are 2 x10^24 molecules
A 0. 784 g sample of magnesium is added to a 250 ml flask and dissolved in 150 ml of water. Magnesium hydroxide obtained from the reaction required 215. 0 ml of 0. 300 m hydrochloric acid to completely react. If one mole of hcl reacts with one mole of hydroxide, how many moles of hydroxide must have been produced for every mole of mg that reacted?.
Answers
0.03225 moles of hydroxide must have been produced for every mole of mg that reacted.
What is the purpose of hydroxide?The ability of sodium hydroxide to change lipids makes it helpful. It is a primary component of home goods including liquid drain cleaners and soap. The most common forms of sodium hydroxide for sale are white pellets or a solution in water.
Briefing:The chemical reaction involving Mg(OH)2 and HCl is:
Mg(OH)2 + 2HCl --> MgCl2 + 2H2O
As a result, we can see that 1 mole of Mg reacts with every 2 moles of HCl.
Calculating for moles HCl:
moles HCl
= 0.300 M * 0.215 L
moles HCl
= 0.0645 mol
The moles Mg then is:
moles Mg = 0.0645 mol * (1 / 2)
moles Mg = 0.03225 mol
To know more about Hydroxide visit:
https://brainly.com/question/21904397
#SPJ4
The driest desert on Earth is the several years to not receive any rainfall. desert. There it has been recorded for O Atacama Sahara (T) Antartic ( Gobi
Answers
Answer:
Atacama
Explanation:
A total of 1.436 F of electricity (1 F=1 mol e−) was required to electrodeposit all of the Zn and Co from a solution of ZnSO4 and CoSO4. The mixture of Zn and Co that was deposited had a mass of 43.57 g. Calculate the masses of ZnSO4 and CoSO4 present in the original solution.
Answers
There were approximately 128.94 g of ZnSO4 and 109.34 g of CoSO4 present in the original solution.
What is electroplating?Electroplating is the process of coating a metal object with a thin layer of another metal by means of electrolysis. In an electrolytic cell with a solution of a salt of the metal to be deposited, the item to be plated is made the cathode (negative electrode).
The electroplating of Zn and Co from the solution involves the transfer of electrons from the cathode to the metal ions in the solution, which results in the deposition of the metals on the cathode. The amount of electricity required for this process is proportional to the amount of metal ions present in the solution, which in turn is proportional to the mass of the metals deposited.
Let's first calculate the moles of electrons transferred in the electroplating reaction:
1.436 F × (1 mol e⁻/1 F) = 1.436 mol e⁻
Since the number of electrons transferred is the same for both Zn and Co, the ratio of the moles of Zn and Co deposited should be the same as the ratio of their atomic masses. The atomic masses of Zn and Co are 65.38 g/mol and 58.93 g/mol, respectively, so the ratio of their masses is:
65.38 g/mol ÷ 58.93 g/mol ≈ 1.11
This means that for every 1.11 moles of Zn deposited, 1 mole of Co is deposited.
Let's assume that x moles of ZnSO4 and y moles of CoSO4 were present in the original solution. Then we can set up the following equations based on the balanced electroplating reaction:
2 e⁻ + Zn²+ → Zn (s)
2 e⁻ + Co²+ → Co (s)
The total number of moles of electrons transferred in the electroplating reaction is:
1.436 mol e⁻ = 2 mol e⁻/mol Zn × x mol ZnSO4 + 2 mol e⁻/mol Co × y mol CoSO4
Simplifying and solving for y:
y = (1.436 mol e⁻ - 2 mol e⁻/mol Zn × x mol ZnSO4) / (2 mol e⁻/mol Co)
y = 0.718 mol CoSO4
Since the ratio of the moles of Zn to Co deposited is 1.11, we can calculate the moles of ZnSO4 from the moles of CoSO4:
x = (1.11 mol Zn/mol Co) × (0.718 mol CoSO4) = 0.798 mol ZnSO4
Finally, we can calculate the masses of ZnSO4 and CoSO4:
mass of ZnSO4 = 0.798 mol × 161.47 g/mol = 128.94 g
mass of CoSO4 = 0.718 mol × 152.06 g/mol = 109.34 g
To know more about cathode, visit:
https://brainly.com/question/4052514
#SPJ1
atomic mass of oxygen according to conventional scale is 16u. if we change the scale from 1/12 to 1/3 then its atomic mass will be
Answers
Answer:
The new atomic mass of oxygen is 64u
Explanation:
Given
Taking the mass by part on the scale of 1/12 gives atomic mass of oxygen = 16 u
Now, when the scale is changed from 1/12 to 1/3, the new mass would become
\(\frac{16}{\frac{1}{12} } * \frac{1}{3} \\= \frac{16 * 12}{3} \\= 64\)
The new atomic mass of oxygen is 64u
Which additional claim can be BEST supported by the evidence from the skulls in the whale fossil record?
A.
The fossil record shows differences in the skulls of the mammals, which shows that they are from three separate species that all lived at the same time.
B.
The fossil record shows similarities in the skulls of the mammals, which shows that they must have had similar habitats and environments.
C.
The fossil record shows that some mammals evolved so that their nostrils are further at the top of their skull for better breathing while in the water.
D.
The fossil record does not give enough evidence about the mammals to determine if there is a relationship between the three skulls.
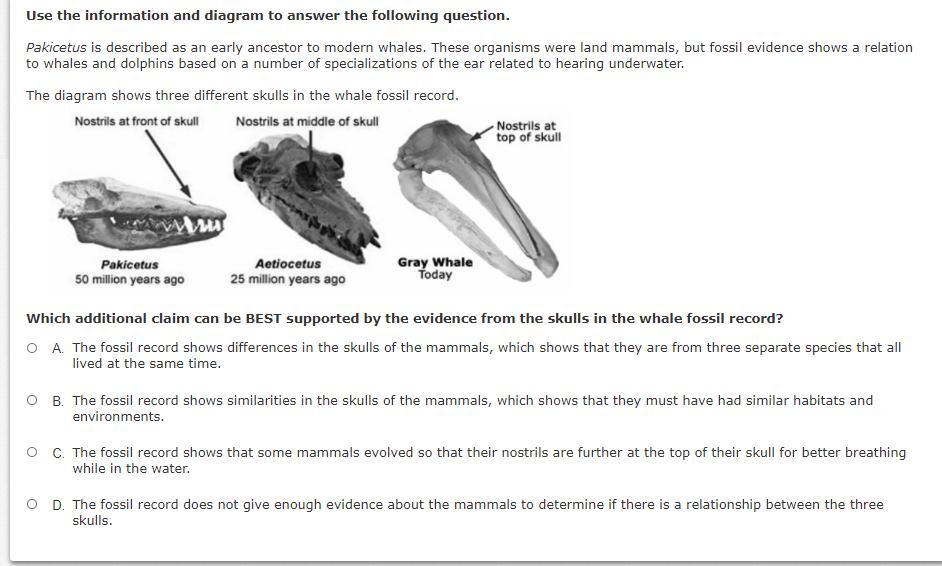
Answers
Answer:
d
Explanation:
Answer:
B
Explanation:
I think it is B because it starts out with it's nostrils so it is on the mouth causing it not to be able to breathe but over time it's nostril moved to the top of the head so they could breather better
how refrigerator magnets work
Answers
Answer:
Explanation:
Fridge magnets are made of weakly ferromagnetic ceramics like barium ferrite or strontium ferrite. The magnetic field created by the fridge magnet aligns the spins of unpaired electrons in metal atoms in the fridge in such a way that the magnet and the fridge door are attracted to each other; this force keeps the magnet stuck to the fridge.
Answer:
the fridge is a magnet
Explanation:
magnets connect to fridge
Consider the reaction, CS2(l)+3O2(g)→CO2(g)+2SO2(g) . The rate of change of CS2(g) is – 0.012 M/s . What is the rate of change of SO2(g) ?
Answers
The rate of change of \(SO_2\)(g)=0.024M/s when the rate of change of \(CS_2(g)\) is – 0.012 M/s.
What is the rate of reaction?Reaction rate, in chemistry, is the speed at which a chemical reaction proceeds.
\(CS_2(l)+3O_2(g)\) → \(CO_2(g)+2SO_2(g)\)
\(Rate = \frac{-dcs_2}{dt} =\frac{-do_2}{dt} =\frac{+dco_2}{dt} =\frac{1dso_2}{dt}\)
Rate = \frac{-dcs_2}{dt} =\frac{-do_2}{dt} =\frac{+dco_2}{dt} =\frac{1dso_2}{dt}
Given:
\(\frac{-dcs_2}{dt} =-0.012 M/s\)
\(\frac{-dcs_2}{dt} =\frac{dso_2}{dt}\)
\(\frac{dso_2}{dt}=0.024M/s\)
The rate of change of \(SO_2\)(g)=0.024M/s
Hence, the rate of change of \(SO_2\)(g)=0.024M/s
Learn more about the rate of change here:
https://brainly.com/question/13103052
#SPJ1
which of the following is the chemical formula for ozone? a) no3 b) so3 c) o2 d) co2 e) o3
Answers
The chemical formula for ozone is O₃. Hence, the correct option is e.
Ozone is a triatomic molecule composed of three oxygen atoms (O₃). It is a pale blue gas with a pungent odor. Ozone plays a vital role in both the Earth's atmosphere and in various industrial and medical applications.
The chemical formula for ozone is O₃, consisting of three oxygen atoms bonded together. Ozone is an allotrope of oxygen and is commonly known for its strong oxidative properties and its role in the ozone layer of the Earth's atmosphere.
Ozone is also generated in the lower atmosphere through various chemical reactions involving pollutants emitted by human activities. High levels of ground-level ozone can cause respiratory issues, irritate the eyes, and harm plant life.
Learn more about Ozone from the link given below.
https://brainly.com/question/27911475
#SPJ4
What is the electron configuration for Fe?
A. [He] 4s2 3d6
B. [Ne] 4s2 3d6
C. [Ar] 4s2 3d6
D. [Ar] 4s2 4d6
Answers
Diamond is one of the common crystalline forms of _____ in which each atom is bonded to 4 others by strong, _____ bonds to create a large 3-D array.
Answers
Diamond is one of the common crystalline forms of carbon in which each atom is bonded to 4 others by strong, covalent bonds to create a large 3-D array.
Diamond is one of the common crystalline forms of carbon. It is an allotrope of carbon, meaning it is a different form or structure of the same element. In diamond, each carbon atom is bonded to four neighboring carbon atoms, forming a three-dimensional lattice structure. These bonds are strong and covalent in nature, involving the sharing of electrons between atoms. The covalent bonds in diamond create a rigid and tightly-packed arrangement of carbon atoms, resulting in its exceptional hardness and durability. Due to the tetrahedral arrangement of carbon atoms, diamond possesses a high degree of symmetry and regularity in its crystal structure. This large, well-organized 3-D array of carbon atoms gives diamond its characteristic properties, such as its transparency, high thermal conductivity, and optical brilliance. Overall, diamond's unique structure and bonding make it one of the most prized and valuable gemstones in the world.
For more information on diamond visit https://brainly.com/question/32700996
#SPJ11
What is the relative atomic mass for pivotium?

Answers
The answer can not be calculated because of lack of available information.
What is atomic mass?
The atomic mass of an element is the average mass of an atom of that element, based on the atomic masses of its isotopes and their relative abundances. The atomic mass of an element is typically expressed in atomic mass units (amu), which are defined as one-twelfth the mass of a carbon-12 atom. The atomic mass of an element is not the same as the mass of a single atom of that element. Instead, it is an average value that takes into account the relative abundances of the different isotopes of the element. The atomic mass of an element is important for understanding the properties of that element and for performing chemical calculations. The atomic mass of an element is used to determine the relative masses of atoms and molecules and is a key factor in determining the chemical behavior of an element.
According to the problem:
The relative atomic mass of Pivotium is the average atomic mass of the element, taking into account the relative abundances of its isotopes.
To calculate the relative atomic mass of Pivotium, the atomic mass of each isotope and the percentage of each isotope in the mixture has to be known.
According to the given information, the mixture contains 27% Pivotium-425 and 73% Pivotium-415.
Use the following formula to calculate the relative atomic mass of Pivotium:
Relative atomic mass of Pivotium = (percentage of Pivotium-425 * atomic mass of Pivotium-425) + (percentage of Pivotium-415 * atomic mass of Pivotium-415)
Since the atomic mass of Pivotium-425 and Pivotium-415 are unkown, this formula to calculate the relative atomic mass of Pivotium is of no use. However, this formula shows how the relative atomic mass of an element is calculated based on the atomic masses of its isotopes and their relative abundances.
It's important to note that the relative atomic mass of an element is a weighted average of the atomic masses of its isotopes, taking into account the relative abundances of each isotope in the element. The relative atomic mass is a measure of the average mass of the atoms in an element and is used to determine the atomic mass of an element in chemical calculations.
To know more about atomic mass, visit:
https://brainly.com/question/29313021
#SPJ1
what do you think will happen to the temperature if you use 75 ml of hcl and 75 ml of naoh instead of the 50 ml each that you used in experiment 1?
Answers
The thing that will happen to the temperature if you use 75 ml of HCl and 75 ml of NaOH instead is that it increases as more heat is released.
How to explain the reaction?A neutralization occurs when NaOH and HCl are combined. Each chemical reaction has a unique heat of reaction, which is the heat exchanged during the process.
The reaction in issue is exothermic (emits heat), and the amount of heat emitted relies on the chemicals used, their concentrations, and the working temperature (it is produced at a low temperature).
The fluctuation in temperature and the mass used determine how much heat is allocated.
In this case, the thing that will happen to the temperature if you use 75 ml of HCl and 75 ml of NaOH instead is that it increases as more heat is released.
Learn more about experiments on:
https://brainly.com/question/14455989
#SPJ1
the structure of the nacl crystal forms reflecting planes 0.541 nm apart. what is the smallest angle, measured from these planes, at which constructive interference of an x-ray beam reflecting off the two planes is observed? assume x-rays of wavelength 0.0649 nm are used? give your answer in degrees.
Answers
The smallest angle, measured from the reflecting planes, at which constructive interference of an X-ray beam is observed is approximately 27.2 degrees.
To determine the smallest angle of constructive interference, we can use Bragg's Law, which states that constructive interference occurs when the path difference between two waves is equal to an integer multiple of the wavelength. The formula is given as:
2d sin(θ) = nλ
Where:
d is the distance between the reflecting planes (0.541 nm)
θ is the angle between the incident X-ray beam and the planes (the desired angle)
n is the order of the interference (we are considering the first-order, so n = 1)
λ is the wavelength of the X-ray beam (0.0649 nm)
Rearranging the formula, we get:
sin(θ) = (nλ) / (2d)
θ = arcsin((nλ) / (2d))
Plugging in the values, we have:
θ = arcsin((1 * 0.0649 nm) / (2 * 0.541 nm))
θ ≈ 27.2 degrees
Therefore, the smallest angle at which constructive interference is observed is approximately 27.2 degrees.
To learn more about constructive interference, here
https://brainly.com/question/31857527
#SPJ4
*
Which galaxy is represented in the
image above?
A. Irregular galaxy
B. Spiral galaxy
C. Elliptical galaxy
D. Electromagnetic galaxy

Answers
Answer:
B! SPIRAL CAUSE ITS SPINNY AND PRETTY UWUUUUUU hehe :3
(Please!!!) Which of the following is not an example of kinetic energy? (2 points) sound chemical energy radiant energy heat
Answers
Answer:
heat
Explanation:
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
A 13. 5 liter balloon is heated from 248 K to 324 K. What will its new
volume be?
Answers
The new volume of the balloon will be determined by the Ideal Gas Law.
What is Ideal Gas Law?The Ideal Gas Law is a mathematical equation used to describe the behavior of an ideal gas under a given set of conditions. It states that, at a constant temperature and pressure, the pressure, volume and amount of gas present are all directly proportional.
This law states that the product of the pressure, volume, and temperature of a gas is a constant.
Since the pressure of the balloon remains constant, the new volume is inversely proportional to the temperature.
Therefore, the new volume can be calculated by dividing the original volume (13.5 liters) by the ratio of the original temperature (248 K) to the new temperature (324 K),
which is 248/324 = 0.76.
The new volume of the balloon is then 13.5 liters / 0.76 = 17.76 liters.
To learn more about Ideal Gas Law
https://brainly.com/question/15882021
#SPJ4
where are people mostly exposed to chemicals?
Answers
Answer:
people are mostly exposed to chemicals through their nose, mouth, eyes, and ears
Explanation:
they are the easiest way for anything, bad or good, to enter the body because people touch their face alot.
This might not answer what you were looking for but the other answer covered it pretty well so I thought I'd give you another angle to the question!
Hope this helps!!
to what volume in ml should you dilute 100ml of a 2.50 m CaCl2 solucion to obtain 0.75 M CaCl2 solution
Answers
GIVEN :
• Volume ,(V1)= 100ml
• Concentration ,(C1) = 2.50 M
• Concentration ,(C2) = 0.75 M
• Volume ,(V2) = ?
The relationship between Volume and Concentration can be represented by formula :
\(C_{1\text{ }}V_1=C_2V_2\)Replacing the given parameters into the formula above , Final Volume (V2) will be :
\(\begin{gathered} C_1V_1=C_2V_2 \\ \therefore V_{2\text{ }}=\text{ }\frac{C_1\cdot V_1}{C_2} \\ \text{ =}\frac{2.50\text{ M }\cdot100\text{ ml}}{0.75\text{ M }} \\ \text{ = }333.33\text{ ml} \end{gathered}\)This means thea final volume = 333.33mldesign a synthesis that would convert phenol primarily to ortho-bromophenol
Answers
In order to convert phenol primarily to ortho-bromophenol, we can use a method called electrophilic aromatic substitution. This involves adding an electrophile to the aromatic ring of the phenol, which will replace one of the hydrogen atoms and result in the formation of a substituted product.
One way to achieve this is by using bromine as the electrophile. We can start by adding bromine water to the phenol, which will form a complex with the bromine. Next, we can add a strong acid such as hydrochloric acid to protonate the phenol and make it more reactive. This will help to generate the electrophile, which can then attack the ortho position of the aromatic ring.
To ensure that ortho-bromophenol is formed primarily, we can control the reaction conditions by using a mild temperature and carefully controlling the pH of the reaction mixture. By doing this, we can prevent the formation of unwanted by-products such as para-bromophenol and meta-bromophenol.
In summary, to convert phenol primarily to ortho-bromophenol, we can use electrophilic aromatic substitution with bromine as the electrophile, and control the reaction conditions to promote ortho selectivity. This synthesis can be carried out in a laboratory setting, and is an important step in the preparation of various organic compounds.
To know more about electrophile visit:
https://brainly.com/question/29789429
#SPJ11
#11
11. Kaitlyn wants to leave a 15% tip for a
restaurant bill of $42.76. How much should she
leave as a tip?
Answers
How is the electronegativity trend related to the first ionization energy trend
Answers
Answer:b
Explanation:
i took the test
Using the equation: 3 NO2(g)H2O-->2 HNO3(aq)+ NO (g)If you start with 10.0 g of NO2(g) and 30.0 g of H2O0):a. What is the limiting reagent for this reaction?b. How many grams of nitric acid can be produced? (theoretical yield)
Answers
Answer:
a. The limiting reagent is NO2.
b. 126g of nitric acid can be produced.
Explanation:
1st) It is necessary to write the balanced equation:
\(3NO_2+H_2O\text{ }\rightarrow2HNO_3\text{ + NO}\)From the balanced equation we know that 3 moles of NO2 (3x46g= 138g) react with 1 mol of water (18g/mol) to produce 2 moles of nitric acid (2x63g= 126g) and 1 mol of NO (30g/mol).
2nd) To calculate the limiting reagent it is necessary to use the given values and the stoichiometry of the balanced equation:
\(\begin{gathered} 138gNO_2-18gH_2O \\ 10.0gNO_2-x=\frac{10.0gNO_2\cdot18gH_2O}{138gNO_2} \\ x=1.30gH_2O \end{gathered}\)The 10.0g of NO2 will need 1.30g of H2O to react.
\(\begin{gathered} 18gH_2O-138gNO_2 \\ 30.0gH_2O-x=\frac{30.0gH_2O\cdot138gNO_2}{18gH_2O} \\ x=230gNO_2 \end{gathered}\)The 30.0g of H2O will need 230g of NO2 to react.
So, as we only have 10.0g of NO2 and 30.0g of H2O, the limiting reagent will be NO2.
3rd) Now, to calculate the theorical yield, we need to use the stoichiometry of the balanced equation using the limiting reagent:
1 mol of H2O produces 2 moles of nitric acid. With the molar mass of nitric acid (63g/mol), we can calculate the grams.
\(2\text{moles}\cdot(\frac{63g}{1\text{mol}})=126g\)
Finally, 126g of nitric acid will be produced if the reaction is 100% efficient (theoretical yield).
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
A pan containing 20.0 grams of water was allowed to cool from a temperature of 95.0 °C. If the amount of heat released is 1,200 joules, what is the approximate final temperature of the water? (5 points)
75 °C
78 °C
81 °C
87 °C
Answers
Answer:
78° C.
Explanation:
The final temperature of water is 81℃ , if it is allowed to cool from a temperature of 95 and it releases heat of 1200J.
What is the final temperature of water?In the following question we will apply specific heat Capacity equation
q=mct Δt
q= 1200J m= 20 g c= 4.186 T₁= 95 ℃
1200= 20 ✕4.186(95-T₂)
T₂=81℃
Hence, The water cools to 81℃ temperature
Learn about specific heat capacity
https://brainly.com/question/1747943
#SPJ2
Pls help me I don’t know how to do this

Answers
Explanation:
We have a 63.9 g sample of calcium hydroxide. First we have to convert those grams into moles. To do that we have to use the molar mass of calcium hydroxide.
Calcium hydroxide = Ca(OH)₂
molar mass of Ca = 40.08 g/mol
molar mass of O = 16.00 g/mol
molar mass of H = 1.01 g/mol
molar mass of Ca(OH)₂ = 1 * 40.08 g/mol + 2 * 16.00 g/mol + 2 * 1.01 g/mol
molar mass of Ca(OH)₂ = 74.10 g/mol
mass of Ca(OH)₂ = 63.9 g
moles of Ca(OH)₂ = 63.9 g /(74.10 g/mol)
moles of Ca(OH)₂ = 0.862 moles
In 1 molecule of Ca we have 2 atoms of O. So in 1 mol of Ca(OH)₂ we will have 2 moles of O atoms.
1 mol of Ca(OH)₂ = 2 moles of O atoms
moles of O atoms = 0.862 moles of Ca(OH)₂ * 2 moles of O /1 mol of Ca(OH)₂
moles of O atoms = 1.724 moles
One mol is similar to a dozen. When we say that we need a dozen eggs we know that we need 12 eggs. If we want a mol of eggs, we want 6.022*10^23 eggs. So one mol of something is 6.022 * 10^23 of that.
1 mol of O atoms = 6.022 * 10^23 atoms
n° of O atoms = 1.724 moles * 6.022 * 10^23 atoms/1 mol
n° of O atoms = 1.04 * 10^24 atoms
Answer: In a 63.9 g sample of Ca(OH)₂ we have 1.04 *10^24 atoms of oxygen.
when a parcel of air sinks in the atmosphere, there is usually _________.
Answers
difference between soap and detergent
Answers
Soap is potassium or sodium salts of a carboxylic acid attached to a long aliphatic chain. Detergent is the potassium or sodium salts of a long alkyl chain ending with a sulfonate group.
What is the ground state electron configuration for the He+2 ion? Select the correct answer below: (σ1s)2(σ∗1s)2 (π1s)2(π∗1s)2 (π1s)2(π∗1s)1 (σ1s)2(σ∗1s)1
Answers
The ground state electron configuration for the He+2 ion is (σ1s)²(σ∗1s)²
The He+2 ion is formed by removing two electrons from the helium atom, which has the electron configuration 1s².
The ground state electron configuration for the He+2 ion can be obtained by removing two electrons from the helium atom's electron configuration. Thus, the ground state electron configuration for the He+2 ion is:
(σ1s)²(σ∗1s)²
Here, the first two electrons occupy the bonding molecular orbital (σ1s), while the next two electrons occupy the corresponding antibonding molecular orbital (σ∗1s).
Therefore, the correct answer is (σ1s)²(σ∗1s)²
Learn more about ground state electron here:
https://brainly.com/question/29594474
#SPJ11