The products of the chlor-alkali process, Cl₂ and NaOH, are kept separated.(b) ClO⁻ or ClO₃⁻ may form by disproportionation of Cl₂ in basic solution. What determines which product forms?
Answers
To produce ClO⁻ and ClO₃⁻, the mole ratio of Cl₂ is 1 :2 and 1 : 2.
What is Disproportionate reaction ?The reaction for disproportionate of Cl₂ in basic medium to produce ClO⁻ and ClO₃⁻ is:
Cl₂ (g) + 2OH⁻ (aq) → Cl⁻(aq) + ClO⁻ (aq) + H₂O(l)
3Cl₂ (g) + 6OH⁻ (aq) → 5Cl⁻ (aq) + ClO₃⁻ (aq) + 3H₂O (l)
When ratio of Cl₂ is 1: 2 then ClO⁻ is produced. Ratio is 3 : 6 then ClO₃⁻ is produced.
To produce ClO⁻ ; the mole ratio of Cl₂
= \(\frac{1}{2}\)
= 1 : 2
To produces ClO₃⁻ ; mole ratio of Cl₂
= \(\frac{3}{6}\)
= 1 : 2
Thus from the above conclusion we can say that To produce ClO⁻ and ClO₃⁻, the mole ratio of Cl₂ is 1 :2 and 1 : 2.
Learn more about the Chlor-Alkali Process here: https://brainly.com/question/11945425
#SPJ4
Related Questions
Arrange oxygen, sulfur, calcium, rubidium, and potassium in order of decreasing electronegativity.Select one:A. O > S > Ca > Rb > KB. O > S > Ca > K > RbC. O > S > Rb > K > CaD. O > S > Rb > Ca > KE. None of these choices are correct.
Answers
The order of decreasing electronegativity is O > S > Ca > Rb > K. The correct option is A.
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a covalent bond. Oxygen has the highest electronegativity value (3.44) on the Pauling scale, followed by sulfur (2.58), calcium (1.00), rubidium (0.82), and potassium (0.82).
Therefore, the order of decreasing electronegativity is O > S > Ca > Rb > K. This is because oxygen and sulfur are both nonmetals with high electronegativity values due to their small atomic size and strong nuclear charge. Calcium is a metal with a lower electronegativity value, followed by the alkali metals rubidium and potassium with even lower values.
It is important to note that electronegativity values are not always strictly decreasing down a group or across a period of the periodic table, but can have some irregularities due to variations in atomic size, nuclear charge, and electron shielding. Option A. is correct answer.
To know more about periodic table refer here:
https://brainly.com/question/17762711#
#SPJ11
What happens when a solid is exposed to sub-zero temperatures?
Answers
Answer:
A substance may undergo phase change from a solid to a gas or from a gas to a solid if the temperature it is exposed to is changed very quickly. If the temperature around a solid is raised very quickly, it can sublimate, or phase change from a solid to a gas without existing as a liquid.
Explanation:
Hypothesis: If a material undergoes a
chemical change, then it will not retain its
original properties because a new substance
is formed.
To test the hypothesis above, you will observe the
changes during the experiment.
To do this, you will use these observations to
compare the___
of the
substances
to the__
substances.
of the

Answers
Answer:
I hate to not answer and have you repost this if you could repost it with the choices by clicking the arrow I can figure it out a lot faster and I'll copy and paste to show you that it's right
Explanation:
I'm good with history biology sum math so if you want to do what I asked and reposted I can give you the answers and I will show that they are correct I won't just guess like some people do just to get points cuz I don't care about points I just get on here to help people
Answer: The answer for the blanks is initial appearance and than final appearance.
Explanation:
How many of the following are WEAK acids?
HNO2 HF HNO3 H2PO4^-
a. 0
b. 1
c. 4
d. 2
e. 3
Answers
The weak acids are HNO₂ and HF. Option D is correct.
HNO₂ (nitrous acid) and HF (hydrofluoric acid) are considered weak acids because they only partially dissociate in water, resulting in a relatively low concentration of H⁺ ions in solution. On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are strong acids, which fully dissociate in water, producing a high concentration of H⁺ ions.
On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are both strong acids;
HNO₃ is a strong acid that fully dissociates in water, resulting in a high concentration of H⁺ ions.
H₂PO₄⁻ is a weak acid in its conjugate acid form (dihydrogen phosphate), but as H₂PO₄⁻, it acts as a weak base rather than a weak acid.
Hence, D. is the correct option.
To know more about weak acids here
https://brainly.com/question/32730049
#SPJ4
PLEASE HELPPPP IM GIVING THE TEST RN
What type of reaction is shown in the equation below? 2H20 - 2H2 + O2
A synthesis
B. decomposition
c. single replacement Will
D. double replacement li
Answers
Answer:
answer is c
Explanation:
single replacement
How many electrons must be gained by nitrogen, N, to achieve a stable electron
configuration?
Answers
Answer:
3 electrons
Explanation:
Nitrate needs 3 electrons to achieve a stable electron configuration
Three is the answer. it needs three to complete its shell
Elements are organized on the periodic table based on their properties. Which statement correctly predicts and explains the chemical reactivity of two metals?
Answers
Lithium (Li) is less reactive than potassium (K) because the valence electrons in lithium atoms are closer to the nuclei and harder to remove. Option D
What is the periodic table?The periodic table has to do with an arrangement of the elements in their order of reactivity. We know that the periodic table is arranged in groups and periods. The groups are the vertical portions while the periods are the horizontal portions.
Given that the reactivity would depend on the position of an element in the periodic table, we would look at where the elements that are to be considered are found in the periodic table.
We know that the elements that occur down the group for the metals are more reactive because they are more shielded thus they leave the attractive force of the nucleus much more easily.
Learn more about periodic table:https://brainly.com/question/11155928
#SPJ1
Missing parts;
Elements are organized on the periodic table based on their properties. Which statement correctly predicts and explains the chemical reactivity of two different metals? Barium (Ba) is less reactive than calcium (Ca) because the valence electrons in calcium atoms are farther from the nuclei and harder to remove. Strontium (Sr) is more reactive than magnesium (Mg) because the valence electrons in strontium atoms are farther from the nuclei and harder to remove. Rubidium (Rb) is more reactive than sodium (Na) because the valence electrons in sodium atoms are closer to the nuclei and easier to remove. Lithium (Li) is less reactive than potassium (K) because the valence electrons in lithium atoms are closer to the nuclei and harder to remove.
calculate the ph of a 500-ml solution to which has been added 20 ml of 100 mm glycinamide hydrochloride
Answers
Being a weak acid (Ka = 5.6x104), the nitrous acid reacts with NaOH as follows: NaOH (l) + HNO2 = NaNO2 (aq) + H2O.
A 0.15 m naoh solution is used to titrate 100 ml of 0.15 m nitrous acid (HNO2). The pH of the initial solution is 2.04, 3.85 for the equivalence point, 8.06 for the point at which 80.0 ml of the base has been added, and 11.56 for the point at which 105 ml of the base have been added. There is initially simply a 0.12M HNO2 solution. Like Ka is: Ka is equal to [H+] [NO2]/[HNO2]. When the ions [H+] and [NO2] come from the same equilibrium, [H+] = [NO2] = x, 5.6x10⁻⁴ = X² / 0.15M. 8.4x10⁻⁵ = X². X = [H⁺] = 9.165x10⁻³M. Since pH = -log [H+], pH = 2.04.
Learn more about pH here:
https://brainly.com/question/29816478
#SPJ4
A.)Place the following in order of decreasing standard molar entropy.
NaCl(s) Na3PO4(aq) NaCl(aq)
a.NaCl(aq) > Na3PO4(aq) > NaCl(s)
b. NaCl(aq) > NaCl(s) > Na3PO4(aq)
c. Na3PO4(aq) > NaCl(aq) > NaCl(s)
d. NaCl(s) > NaCl(aq) > Na3PO4(aq)
e. NaCl(s) > Na3PO4(aq) > NaCl(aq)
Answers
The standard molar entropy is a measure of the disorder or randomness of a substance at standard conditions. Generally, solids have lower entropy than liquids, and liquids have lower entropy than gases.Option e is correct.
NaCl(s) Na3PO4(aq) NaCl(aq)
a.NaCl(aq) > Na3PO4(aq) > NaCl(s)
b. NaCl(aq) > NaCl(s) > Na3PO4(aq)
c. Na3PO4(aq) > NaCl(aq) > NaCl(s)
d. NaCl(s) > NaCl(aq) > Na3PO4(aq)
e. NaCl(s) > Na3PO4(aq) > NaCl(aq)
Based on the principles mentioned above, option e is correct. NaCl(s) has the lowest entropy because it is a solid, while Na3PO4(aq) has a higher entropy because it is in aqueous solution, and NaCl(aq) has the highest entropy since it is a more disordered state than both solid NaCl and Na3PO4(aq).
So, the correct order is NaCl(s) > Na3PO4(aq) > NaCl(aq).
For more such questions on entropy
https://brainly.com/question/3465355
#SPJ11
In chemistry class, Allen determined the effectiveness of various metals in releasing hydrogen gas from hydrochloric acid. Several weeks later, Allen read that a utilities company was burying lead next to iron pipes to prevent rusting. Allen hypothesized that less rusting would occur with the more active metals. He placed the following into 4 separate beakers of water: (a) 1 iron nail, (b) 1 iron nail wrapped with an aluminum strip, (c) 1 iron nail wrapped with a magnesium strip, and (d) 1 iron nail wrapped with a lead strip. He used the same amount of water, equal amounts (mass) of the metals, and the same type of iron nails. At the end of 5 days, he rated the amount of rusting as small, moderate, or large. He also recorded the color of the water. What is the independent variable?
a) the amount of water
b) the metals strips
c) amount of rust
d) hydrochloric acid
Answers
Answer:
b) the metals strips
Explanation:
In an experimental design, an independent variable is a variable that is changed or manipulated in a series of experiments. An independent variable is not dependent on any other variable in the experiment. The hypothesis for this experiment is stated to be: "If the chemical activity of the metallic wrapper is increased, then less rusting of iron will occur. The independent variable relates to the type of metal wrapping strip, and the dependent variables are the amount of rusting and color of the water.
the Earth and moon have different forces of gravity.
Which measurement did they not use? *
mass
density
weight
O
volume
Answers
Answer:
volume
Explanation:
the mass is the same on earth as it is on the surface of the moon. The weight is determined by the force of gravity. The density is the thickness. But the volume is not a factor.
Can somebody plz answer both questions correct!!!! Only 1-2 sentences per question is fine :)
(WILL MARK BRAINLIEST)...promise :D

Answers
Answer:
1) A compound is the chemical combination of two or more elements into a single substance.
An atom is the smallest particle of an element.
2) Elements have only one kind of atom.
Compound always have more than one kind of atom.
Answer:
1) An atom is the smallest possible piece of a chemical element, and a compound can be composed of two or more separate elements
2) An element is made up of only one kind of atom and a compound is made up of two or more kind of atoms
Explanation:
Plan an investigation to examine the relationship between the amount of gas
and its volume. Identify the materials and write a procedure, identifying the
constants and variables. Conduct the investigation and record your
observations.
I can’t think of anything to use for this experiment and we aren’t told what to use so if anyone had any ideas for that I would really appreciate it
Answers
Answer:
You will need a Kukushili pressure cooker for this experiment. This pressure cooker will allow you to compress the gases and observe its volume.
56.75mL of 0.256 HI M reacts with 10.00mL sample of NaOH, what is the molarity of sodium hydroxide?
Answers
Answer: 1.45M
Explanation:
The definition of molarity is moles/liter. The neutralization of NaOH with HI is:
HI + NaOH = NaI + H2O
One mole of HI reacts with 1 mole of NaOH. We'll assume this is a titration reaction and that the 10.00ml sample of NaOH contains the same number of moles as the 56.75ml of 0.256M HI.
Moles HI: (0.256 moles/liter)*(0.05675 L) = 0.01453 moles HI
That means we muct have 0.01453 moles NaOH in 10.0ml of NaOH solution.
(0.01453 moles NaOH)/(0.010L) = 1.45 M
==
Another approach is to use the relationship M1V1 = M2V2, which is useful for titrations (M is concentration and V is volume):
We want M2, so rearrange: M2 = M1V1/V2
M2 = (0.256M)*(56.75ml)/(10.0ml)
M2 = 1.45M
If a plant has a total of 17g of carbon dioxide and water reacting in photosynthesis, then how much glucose and oxygen will the plant produce?
A. 8.5g
B. 34g
C. 17g
(Take your time)
Answers
Answer:
Basically it would be 8.5g. Not total sure.
Explanation:
If its right plz give brainlest.
Answer:
A. 8.5g
Explanation:
Have a great day!
3. Which two of the following elements would you expect to have a very different melting point than titanium (TI): iron (Fe),
sulfur (S), selenium (Se), or chromium (Cr)? Explain why you chose those two elements.
Answers
The two elements that would have a very different melting point than titanium (Ti) are Sulfur (S) and Selenium (Se)
Periodic tableFrom the question, we are to determine the elements that would have different melting point than titanium.
Titanium (Ti) is a transition metal which belongs to 3d- block on the periodic table.
Iron (Fe) and Chromium are also transition metals; and they belong to the 3d- block on the periodic table. Transition metals have high melting points.
Sulfur (S) and Selenium (Se) are Group 16 elements, which will have lower melting points compared to the transition elements.
Hence, the two elements that would have a very different melting point than titanium (Ti) are Sulfur (S) and Selenium (Se)
Learn more on Periodic table here: https://brainly.com/question/13555712
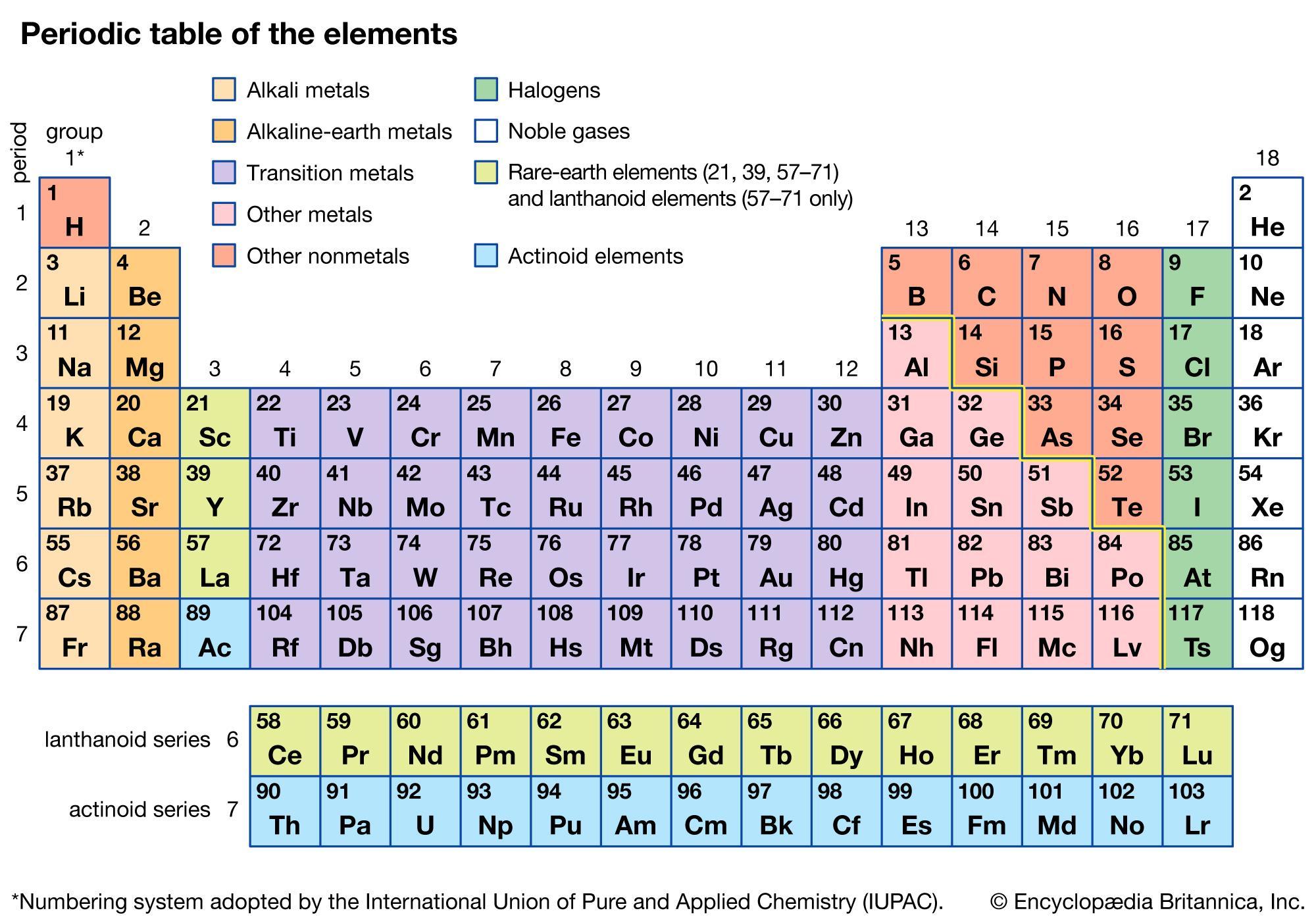
HELP⚠️THANK YOU!!!!!

Answers
what is a complex permanent tissue? mention the major functions of this tissue .
Answers
Answer:
Complex permanent tissue is defined as a collection of structurally dissimilar cells performing a common function or set of functions.
its functions are:
1.help in the transportation of organic material, water, and minerals up and down the plants.
2.it helps in transportation of food from leaves to other parts of plants.
have a great dayyyyyy
why was it necessary to titrate blank samples in vitamin c
Answers
In order to accurately determine the amount of vitamin C present in a sample, it is necessary to titrate blank samples.
This is because the presence of other substances in the sample can interfere with the accuracy of the results. By titrating blank samples, which are essentially samples without vitamin C, we can determine the amount of interference present in the sample.
This interference can then be subtracted from the total amount of titrant used in the sample to obtain the accurate amount of vitamin C present.
Additionally, blank samples are used to ensure that the titrant and other equipment being used are working properly and are not contaminated. Overall, titrating blank samples is a crucial step in obtaining accurate results in the analysis of vitamin C.
To know more about vitamin C refer here: https://brainly.com/question/3594842#
#SPJ11
Which of these statements relating to ecological succession is true?
During succession, there is no change to the physical or chemical environment.
During succession, existing species resist interaction with new species.
During succession, new species move into an area and colonize it.
Most ecological successions occur over 10 to 15 years.
Answers
Answer: During succession, new species move into an area and colonize it.
Explanation: Ecological succession refers to the process of change in the composition and structure of an ecosystem over time. It occurs due to the interactions between the biotic (living) and abiotic (non-living) components of an environment. As succession progresses, new species gradually establish and thrive in the area, leading to a change in the species composition. This process can occur over a long period of time, ranging from decades to centuries, depending on various factors such as environmental conditions and the specific type of succession.
Answer number 5 please if you say for point I will report you:)

Answers
Answer:
A
Explanation:
Lighting a fireworks the potential energy is converted to all forms of energy like light, heat and sound energy
what are the products for c3h8+o2-->co2+h2o
Answers
Answer:
Products would be on the right. Reactants would be on the left
Explanation:
For there to a measurable amount of heat, there must be a change in ____ in the system.
a.Enthalpy
b.Temperature
c.Mass
d.Rate
Answers
b.temperature
in a calorimetric determination, either 1. an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or 2. an endothermic process occurs and heat, q, is positive, indicating that thermal energy is transferred from the surroundings to the system.
mass in grams of 3.32 mol k
Answers
Answer:
129.8 grams
Explanation:
Im a bit confused of what your are asking but if your are asking the mass in grams of K (Potassium) it is very easy dont worry
You multiply mols x Molecular weight.
So
3.32x 39.1= 129.8 grams
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
a mineral sample from rock unit c has 50,000 atoms of
uranium-235 and 150000 atoms of lead 207
Answers
To calculate the ratio of uranium-235 to lead-207 atoms in the mineral sample, we can use the atomic masses and the concept of radioactive decay.
The ratio of uranium-235 to lead-207 atoms in the mineral sample is 1:3.The atomic mass of uranium-235 (U-235) is approximately 235 atomic mass units (amu), while the atomic mass of lead-207 (Pb-207) is approximately 207 amu.
Given:
Number of uranium-235 atoms = 50,000
Number of lead-207 atoms = 150,000
To find the ratio, we divide the number of uranium-235 atoms by the number of lead-207 atoms:
Ratio = Number of uranium-235 atoms / Number of lead-207 atoms
Ratio = 50,000 / 150,000
Simplifying the ratio:
Ratio = 1/3
Therefore, the ratio of uranium-235 to lead-207 atoms in the mineral sample is 1:3.
To learn more about atomic mass click here:brainly.com/question/3187640
#SPJ11
Which substances have Delta. Hf = 0 kJ/mol by definition? Select all that apply. O2(g) N(g) H2O(l) Br2(l) Fe(s) He(g).
Answers
The substances that have Delta Hf = 0 kJ/mol are O2(g), Br2(l), He(g), Fe(s).
What is delta Hf = 0?Delta Hf = 0 is the standard enthalpy of any element in its most stable form is equal to zero.
When enthalpy is negative, the delta Hf is minus zero, which means the system releases heat.
When the system gains heat, the delta Hf is positive.
Thus, the substances are O2(g), Br2(l), He(g), Fe(s).
Learn more about Delta Hf, here:
https://brainly.com/question/25912291
Answer:
1,4,5,6
Explanation:
what percentage of water on earth is fresh water
Answers
Answer:
only 2.5% is fresh water on earth
Answer:
The answer is 3%
Explanation:
what is the heating curve on the horizontal line
Answers
Answer:
The plateaus or horizontal lines on the graph represent the transition between states of the sample. The first plateau represents the melting (or transition from solid to liquid) and the second plateau represents boiling (or transition from liquid to gas).
Explanation:
what evidence have you discovered to explain how the structure of compounds determens the properties of the compounds
Answers
All of the properties such as ice floating on water, while most solids would sink when placed in its liquid are all due to the structure of the compounds.
The structure of the compounds includes the bonding angle, the type of bonds, the size of the molecule, the interactions between the molecules etc. Slight changes in the chemical structure and affect the properties if the compound.
Isomeric compounds with the same chemical formula but different structures can have different melting and boiling point and differ in reactivity and flammability.
Another common change in isomers are with the double bonds. A double bond can be in the cis formation or in the trans formation, and this will affect its properties as trans isomers will be having high melting point than the cis isomer.
Thus, structure of compounds do determine the properties of the compounds.
To know more about the structure and properties of compounds
https://brainly.com/question/4661963
#SPJ1