The purpose of the saturated aqueous sodium chloride wash is to: remove water from the organic layer increase the solubility of the product in the aqueous layer both a and b none of the above
Answers
remove water from the organic layer.
Explanation:
The purpose of the saturated aqueous sodium chloride wash is to remove water from the organic layer. This is done by increasing the ionic strength of the aqueous layer, which causes the water molecules to be preferentially attracted to the salt ions rather than remaining in the organic layer. This results in a more efficient separation of the organic and aqueous layers. So, the correct answer is option A: remove water from the organic layer.
To know more:
https://brainly.com/question/14567984?
#SPJ11
Related Questions
5.27x10^45 molecules of h20 is how many moles
Answers
Answer:
6.02 × 10^23 molecules = 1 mole
5.27 × 10^45 molecules = x
x = 5.27 × 10^45/ 6.02 × 10^23 × 1
= 8.754 × 10^21 mol
I don't know if it's correct but based on the question that was the only way I saw how to work it out
How does the carbonate ion concentration of seawater affect the calcification rate of a coral reef?
Answers
As the concentration of carbonate ions is increased, the speed of calcification increases.
Why is carbonate important for ocean life?
Calcium carbonate minerals are the life blocks for the skeletons and shells of several marine organisms. In areas where most life now congregates within the ocean, the seawater is supersaturated with regard to calcium carbonate minerals.
How do organisms use carbonate?
the most way is via photosynthesis by phytoplankton, where CO2 and sunlight are wont to produce sugars and a waste product of oxygen. carbon are often utilized in the calcium carbonate shells of animals including corals, clams, mussels, oysters.
Learn more about coral reef:
brainly.com/question/10970167
#SPJ4
The attraction between a positive metal ion and the electrons surrounding
it is a(n)
metallic bond
O ionic bond
chemical bond
covalent bond
Answers
Answer:
metallic bond
Explanation:
Metallic bonds are the attraction between a positive metal ion and the electrons surrounding them.
This bond is found in metals and their alloys. They are interatomic forces.
In this bond, a positive nuclei joins with all other closely packed atoms in the lattice and the electron cloud formed by losing their outermost shell electrons. Most of the physical properties of metals is due to this bond type.which nutrients are most responsible for eutrophication?
a. carbon and oxygen
b. carbon and phosphorus
c. nitrogen and oxygen
d. nitrogen and phosphorus
Answers
Answer:
C. Nitrogen and Phosphorous.
Explanation:
Which concept accounts for the whole-number subscripts in chemical formulas?.
Answers
The concept that accounts for the whole-number subscripts in chemical formulas is the law of definite proportions. This law states that a compound is always composed of the same elements in the same proportion by mass, regardless of the source or method of preparation.
The whole-number subscripts in a chemical formula indicate the ratio of atoms of each element in the compound. Let's take an example of the water molecule, H2O, to understand the concept better. In this formula, the subscript 2 indicates that there are two hydrogen atoms for every one oxygen atom.
This means that the ratio of hydrogen atoms to oxygen atoms in a water molecule is 2:1, which is always true for any sample of water, whether it is obtained from a river or produced in a laboratory. The law of definite proportions is a fundamental concept in chemistry because it helps scientists predict and understand the behavior of different compounds.
By knowing the composition of a compound, scientists can determine its properties and use that information to create new materials and substances that are tailored to specific needs.
To know more about accounts visit:
https://brainly.com/question/14138124
#SPJ11
what is used to observe things, like cells, that are too small to see with the naked eye.
Answers
Answer:
a microscope is used to see little things the naked eye can't
calculate the ph after 0.010 mol hcl is added to 225.0 ml of a buffer solution that is 0.10 m ethylamine and 0.15 m ethylammonium nitrate? (ethylamine, kb = 6.4×10-4 )
Answers
The pH after adding 0.010 mol of HCl to 225.0 mL of a buffer solution containing 0.10 M ethylamine and 0.15 M ethylammonium nitrate can be calculated by considering the reaction between HCl and ethylamine to form ethylammonium chloride.
1. Calculate the initial moles of ethylamine in the solution:
Moles of ethylamine = concentration of ethylamine * volume of solution
= 0.10 M * 0.2250 L
= 0.0225 mol
2. Calculate the moles of ethylammonium nitrate in the solution:
Moles of ethylammonium nitrate = concentration of ethylammonium nitrate * volume of solution
= 0.15 M * 0.2250 L
= 0.0338 mol
3. Determine the limiting reagent:
The limiting reagent is the one with fewer moles, which in this case is ethylamine (0.0225 mol).
4. Calculate the moles of HCl added:
Moles of HCl added = 0.010 mol
5. Calculate the moles of ethylamine remaining after the reaction:
Moles of ethylamine remaining = initial moles of ethylamine - moles of HCl added
= 0.0225 mol - 0.010 mol
= 0.0125 mol
6. Calculate the moles of ethylammonium chloride formed:
Moles of ethylammonium chloride formed = moles of HCl added
= 0.010 mol
7. Calculate the new total volume of the solution after adding HCl:
Total volume of the solution = initial volume + volume of HCl added
= 0.2250 L + 0.010 L
= 0.2350 L
8. Calculate the new concentration of ethylamine:
Concentration of ethylamine = moles of ethylamine remaining / total volume of the solution
= 0.0125 mol / 0.2350 L
≈ 0.053 M
9. Calculate the new concentration of ethylammonium nitrate:
Concentration of ethylammonium nitrate = moles of ethylammonium nitrate / total volume of the solution
= 0.0338 mol / 0.2350 L
≈ 0.144 M
10. Write the balanced equation for the reaction between ethylamine and HCl:
\(C_2H_5NH_2\) + HCl → \(C_2H_5NH_3\)+ Cl-
11. Calculate the concentration of hydronium ions (H3O+):
[H3O+] = concentration of ethylammonium chloride
= moles of ethylammonium chloride / total volume of the solution
= 0.010 mol / 0.2350 L
≈ 0.043 M
12. Calculate the pOH:
pOH = -log10([OH-])
= -log10(Kw / [H3O+])
= -log10(1.0 x \(10^{-14\) / 0.043)
≈ 11.30
13. Calculate the pH:
pH = 14.00 - pOH
= 14.00 - 11.30
≈ 2.70
Therefore, the pH after adding 0.
For more such questions on ethylamine, click on:
https://brainly.com/question/30262973
#SPJ11
rank the given compounds in decreasing order of boiling points (from highest to lowest boiling point).
I. CH3CH2CH2CH2OH
II. CH3CH2OCH2CH3 III. CH3OCH3 IV. HOCH2CH2CH2OH a. II > IV > > III b. I> IV> || > III c. IV> | > || > III d. III > || > | > IV e. IV> || > I > III
Answers
The correct ranking of the compounds in decreasing order of boiling points is IV > I > II > III. The correct answer is option (c).
Boiling point is influenced by molecular weight, polarity, and hydrogen bonding. Higher boiling points indicate stronger intermolecular forces between molecules. Comparing the given compounds, the molecule with the strongest intermolecular forces will have the highest boiling point. Therefore, to rank the compounds in decreasing order of boiling points, we need to compare the polarity and hydrogen bonding of each compound.
Compound IV, HOCH2CH2CH2OH, has the highest boiling point because of the presence of two hydroxyl groups that can form hydrogen bonds between molecules.
I, CH3CH2CH2CH2OH, has only one hydroxyl group, but a larger molecular weight than II and III, making it have a higher boiling point.
II, CH3CH2OCH2CH3, is an ether and has a lower boiling point than I and IV due to the absence of a hydroxyl group.
Compound III, CH3OCH3, is nonpolar and cannot form hydrogen bonds, giving it the lowest boiling point among the given compounds.
Therefore, the correct option is (c)
For more such questions on compounds:
https://brainly.com/question/23334479
#SPJ11
This ranking is based on the intermolecular forces present in each compound. Ethylene glycol has the highest boiling point due to strong hydrogen bonding, followed by propanol with hydrogen bonding and dipole-dipole interactions. Acetaldehyde has dipole-dipole interactions, ethyne has weak van der Waals forces, and ethanol has the weakest intermolecular forces among these compounds. Thus, their boiling points decrease in the order given above.
Boiling point is the temperature at which a liquid changes to a gas, and it depends on the intermolecular forces between the molecules. Stronger intermolecular forces lead to a higher boiling point because more energy is required to separate the molecules. In this case, ethylene glycol has the highest boiling point because it has two hydroxyl groups, which can form strong hydrogen bonds with neighboring molecules. Propanol also has hydrogen bonding and dipole-dipole interactions, while acetaldehyde has dipole-dipole interactions. Ethyne has only weak van der Waals forces, and ethanol has the weakest intermolecular forces, which accounts for their lower boiling points.
Learn more about Ethylene glycol here;
https://brainly.com/question/30530800
#SPJ11
An organ system is formed by..
A) two or more cells working together.
B) two or more tissues working together.
C) two or more organs working together.
D) two or more organisms working together
Answers
draw structural formulas for an aldehyde or ketone and alkyl (or aryl) bromide that could be used in a grignard synthesis of the alcohol shown.ch2ch2oh
Answers
Grignard synthesis of the alcohol shown involves the following reaction: CH2CH2Br + Mg + 2(C2H5)2O → CH2CH2MgBr + 2C2H5OHWhen we compare the equation with the reagents available, we can see that it requires CH2CH2Br and two molecules of C2H5OH.
From these, CH2CH2OH is synthesized. As the equation suggests that CH2CH2Br is the alkyl halide used, we can add CH2CH2Br and an aldehyde or ketone as a reactant. To draw the structural formulas for the reaction, follow the below guidelines: Step 1: Add an aldehyde or ketone Aldehydes and ketones are organic compounds containing carbonyl groups. They have the following formula: RCHO (aldehyde) and R2CO (ketone), respectively. An example of an aldehyde is formaldehyde, which has a structural formula HCHO. When we add HCHO to the reaction, the structural formula for the reactant becomes: CH2O.Step 2: Add an alkyl or aryl bromide The next step is to add an alkyl or aryl bromide to the reactant. An alkyl bromide is an organic compound containing a carbon-bromine bond, while an aryl bromide contains a bromine atom attached to an aromatic ring. The simplest example of an alkyl bromide is CH3Br, while the simplest aryl bromide is bromobenzene (C6H5Br). For this reaction, we will add CH2CH2Br as the alkyl bromide. The structural formula for the reactant becomes: CH2CH2Br + CH2OHere is the required structural formula in 100 words. The Grignard synthesis of the alcohol shown in the equation CH2CH2Br + Mg + 2(C2H5)2O → CH2CH2MgBr + 2C2H5OH requires CH2CH2Br and two molecules of C2H5OH. Therefore, we can add CH2CH2Br and an aldehyde or ketone to form the desired alcohol. For this purpose, we will use HCHO as an aldehyde and CH2CH2Br as an alkyl bromide. The structural formula for the reactant will be CH2CH2Br + CH2O.
For more information on Grignard visit:
brainly.com/question/31845419
#SPJ11
02.04 Slide #2 Fill in the blanks based on the videos T Speed of reaction. When the of the reactants are moving too in a chemical reaction, there are fewer between particles. This means there are fewer for particles to correctly. Here you see two particles moving slowly. Though these particles seem ready to react, their slow speeds do not allow them to have the to react in this particular case. Instead, these particles just bounce apart. Particle alignment. If the particles of the reactants in a are not aligned Just right during a collision, they will away from one another. Here you see two particles moving toward each other. However, they are not facing each other in the right way to react. These particles just bounce away from each other.
Answers
Answer:
kylee is the best
Explanation:
Rocks are classified into groups based on how they
Answers
Hope dis helps
Physical properties like hardness, melting point, and boiling point depend on
Answers
Answer:
depend on nature of matter
Answer:
It depends on the temperature and how the molecules react
Which of these ions is most likely to be leached from the soil?
a. magnesium ions,
b. chlorine ions,
c. calcium ions,
d. iron ions
e. potassium ions
Answers
Iron Group of answer choices prevents carbon monoxide from binding to hemoglobin. is the binding site for carbon dioxide on the hemoglobin molecule. is a plasma coagulation factor. is needed to produce hemoglobin. interferes with the normal function of hemoglobin.
Answers
The correct statement from the given Iron Group of answer choices is "interferes with the normal function of hemoglobin.
Carbon monoxide (CO) interferes with the normal function of hemoglobin (Hb) present in red blood cells (RBC). Hemoglobin is a protein that carries oxygen from the lungs to the tissues and carries back carbon dioxide to the lungs for elimination. Carbon monoxide can bind to the heme portion of hemoglobin forming carboxyhemoglobin (HbCO), which reduces the oxygen-carrying capacity of the blood.Red blood cells have a higher affinity for carbon monoxide than for oxygen. This is because carbon monoxide has a binding site similar to that of oxygen. Inhaling carbon monoxide at high concentrations can lead to carbon monoxide poisoning. Symptoms of carbon monoxide poisoning include headache, nausea, vomiting, dizziness, confusion, chest pain, and shortness of breath.
To know more about hemoglobin visit:
https://brainly.com/question/31765840
#SPJ11
Using the phase diagram for H₂O, what phase is water in at 1 atm pressure
and -5°C?
A. It is at its melting point.
B. It is in the gas phase.
C. It is in the liquid phase.
D. It is in the solid phase.
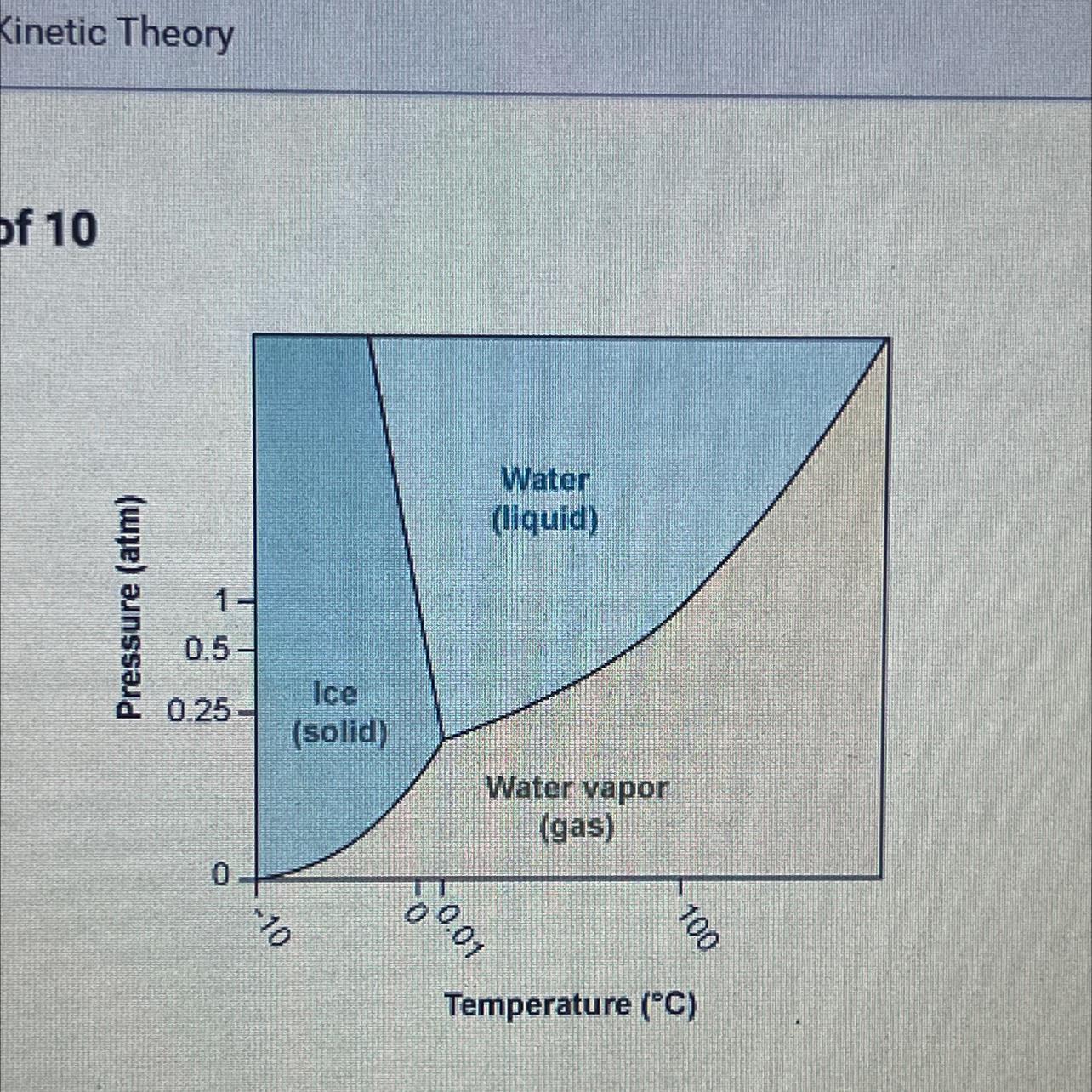
Answers
The number of phases that exist in equilibrium in a system depends upon the variables like temperature, pressure and composition. Here at 1 atm pressure water is in solid phase. The correct option is D.
What is phase diagram?The phase equilibria studies are made simpler by the use of plots which show how the various equilibria depend on the temperature, pressure and composition variables. These diagrams are called phase diagrams.
Here the pressure 1 atm on y-axis coincides with the temperature -5°C on x-axis. The water system is a one component system. So the water is in solid phase.
Thus the correct option is D.
To know more about phase diagrams, visit;
https://brainly.com/question/16945664
#SPJ9
For one process, elect three alloying components for Ti alloys to give alpha-gamma
phase from an eutectoid reaction and another process, give three alloying components added to Ti to give beta phase from a peritectic reaction. Be sure to describe each process in not more than three sentences.
Answers
In both processes, the choice of alloying elements is crucial to control the phase transformations and achieve the desired microstructure and properties in titanium alloys.
To achieve the alpha-gamma phase transformation in titanium alloys via the eutectoid reaction, three alloying components commonly used are aluminum (Al), vanadium (V), and niobium (Nb). These elements form a solid solution with titanium, promoting the formation of the desired phases. The eutectoid reaction occurs when the alloy is cooled from a high temperature, causing the transformation of the high-temperature gamma phase into the low-temperature alpha phase. For the formation of the beta phase in titanium alloys through the peritectic reaction, three commonly used alloying components are molybdenum (Mo), chromium (Cr), and iron (Fe). These elements promote the stabilization of the beta phase at room temperature, inhibiting the transformation to the alpha phase. The peritectic reaction occurs when a small amount of liquid phase reacts with the existing solid phase to form the desired beta phase. The specific compositions and processing conditions can be further optimized based on the intended application and performance requirements.
For such more questions on elements
https://brainly.com/question/26842818
#SPJ8
250.0 mL of a 2.500 M NaOH solution was mixed with 250.0 mL of a 2.500 M HCl solution in a calorimeter. Both the solutions were at the same temperature initially. Determine the heat of the reaction (kJ/mole), if the temperature goes from 2.0 Celsius to 48.8 Celsius. The specific heat of the solution is 4.190 J/goC. Assume a density of 1.025 g/mL.
Please provide it step-by-step. Heat of formation equation = (mass)(specific heat)(change of temp.)
Answers
The reaction has a heat of 161.1 kJ/mol.
What is the molarity of the 250 ml NaOH solution?If 250 mL of a NaOH solution contain 1 milligrams of NaOH, the solution's molarity is 10-4 M. The amount of solute in 1 litre of solution is known as the molarity.
We can use the following formula to get the reaction's heat:
Q = m × c × ΔT
The total volume of the mixture is 500.0 mL because we are aware that the volume of each solution is 250.0 mL:
m = V × ρ
m = 500.0 mL × 1.025 g/mL
m = 512.5 g
The change in temperature must then be calculated:
ΔT = (48.8°C - 2.0°C) = 46.8°C
Assuming that the acid used is HCl and the base used is NaOH, their molar masses are:
HCl: 1 mol of HCl = 36.46 g/mol
NaOH: 1 mol of NaOH = 40.00 g/mol
The reaction between HCl and NaOH has the following chemical formula:
HCl + NaOH → NaCl + H2O
As can be seen, the reaction's stoichiometry is 1:1, which means that 1 mole of HCl combines with 1 mole of NaOH.
So, the following formula can be used to determine the reaction's heat:
Q = m × c × ΔT
Q = 512.5 g × 4.190 J/goC × 46.8°C
Q = 100,697 J or 100.697 kJ
Since both solutions have a 2.500 M molarity, the number of moles of either HCl or NaOH can be determined as follows:
moles = M × V
moles = 2.500 mol/L × 0.2500 L
moles = 0.6250 mol
Therefore, the heat of the reaction is:
Q/mol = Q / moles
Q/mol = 100.697 kJ / 0.6250 mol
Q/mol = 161.1 kJ/mol
To know more about reaction visit:-
https://brainly.com/question/14025220
#SPJ1
3. Given the name: cytidine 5' - diposphate, What is the pentose present? What base is present? How many phosphate groups are present? At what carbon of the pentose does the phosphate group bond?
Answers
Cytidine 5'-diphosphate is an important intermediate in cellular metabolism, particularly in the biosynthesis of RNA and DNA.
Cytidine 5'-diphosphate contains a pentose sugar, a nitrogenous base, and two phosphate groups. The pentose sugar present is ribose, and the base present is cytosine. The phosphate groups present are two. The phosphate group on the 5'-carbon of the pentose sugar is joined to the cytosine base, while the phosphate group on the 3'-carbon of the pentose sugar is joined to another nucleotide in the polynucleotide chain.
The bond between the phosphate group and the pentose sugar occurs at the 5' carbon. Thus, cytidine 5'-diphosphate is an important intermediate in cellular metabolism, particularly in the biosynthesis of RNA and DNA.
To know more about metabolism visit:-
https://brainly.com/question/31384460
#SPJ11
What is the empirical formula of a compound containing 65.5 grams carbon, 5.5 grams hydrogen, and 29.0 grams oxygen?
Answers
Answer: The empirical formula is C₃H₃O.
Explanation: Assume we have 100 g of the compound. Then we have 65.65.5 g of C, 5.5 g of H, and 29.0 g of O.
Answer:
C₃H₃O
Explanation:
I took the test
What is the mass (in grams) of 4.5 x 10^23 units of NaCl?
could you also explain?
Answers
Answer:
43.7 g
Explanation:
unit mass of NaCl = (23 + 35.5) u = 58.5 u
58.5 is the mass of one unit formula of NaCl (since NaCl is not a molecular compound)
1 u = 1.661×10^-24 g
if 1 unit formula of NaCl has 58.5 u, then 4.5×10²³ units of NaCl will have:
58.5 u × (1.661×10^-24 g/1 u) × 4.5×10²³ = 43.7 g
what is the letter in the equation for heat

Answers
Answer:
H is the letter for heat
Balancing the equation below:
Can anyone please help me out with this formula equation.

Answers
Answer:
1: 1, 2, 3
2: 2, 2, 3
3: 2, 1, 2, 1
Explanation:
A simple way to do this:
Take an extra page and write your formula at the top, then take your elements (for example in #1, N and H) and write them on the reactants and products sides. On the reactants side write down the numbers you have (N-2, H-2) and the same for the products side (N-1, H-3). Find your GCF's and then you got your answer. It takes a bit of time to figure out HOW to do it but its a good skill to have. I have attached #1 for you.
If you have questions let me know!
Hope that helps

What does this image represent?
A. Linear molecule with one domain
B. Linear molecule with two domains
C. Tetrahedral molecule with four domains
D. Trigonal planar molecule with three domains

Answers
Answer: A.
Explanation:
Answer:
A. Linear molecule with one domain.
Hope this helps! :)
Explanation:
A covalent molecule Q contains exactly 6 shared electrons. What is Q
Answers
Answer:
oxygen has six shared electrons
Covalent molecules are the group of atoms that shares a covalent bond. Ammonia is a molecule that shares three pairs or six electrons to form a covalent bond.
What are covalent bonds?Covalent bonds are the intermolecular bonds that mutually involve the sharing of electrons between the two or more atoms of the molecules. In ammonia three pairs of electrons are in a covalent bond.
In an ammonia molecule nitrogen have five electrons in its outer shells and hydrogen have one electron each. When three hydrogen shares their electrons with nitrogen to make a covalent bond the octet of the nitrogen atom is completed.
Therefore, covalent molecule Q is ammonia.
Learn more about covalent bond here:
https://brainly.com/question/489487
#SPJ2
a solution is made containing 14.6 g of ch3oh in 184 g of water. calculate the mole fraction of methanol, ch3oh.
Answers
The mole fraction of methanol (CH₃OH) is 0.0427 or 4.27%.
Mole fraction is a measure of the concentration of one substance in a mixture, expressed as the ratio of the moles of the given substance to the total moles of all the substances in the mixture. Mole fraction is an important concept in chemistry, as it allows us to determine the properties of the mixture, such as its vapor pressure, boiling point, and freezing point.
To calculate the mole fraction of methanol (CH₃OH) in the given solution, we must first calculate the moles of methanol present. This is done by dividing the mass of methanol (14.6 g) by its molecular weight (32.04 g/mol).
moles CH₃OH = 14.6 g / 32.04 g/mol = 0.456 mol
We then calculate the moles of water by dividing the mass of water (184 g) by its molecular weight (18.02 g/mol).
moles H₂O = 184 g / 18.02 g/mol = 10.211 mol
The mole fraction of methanol can then be calculated by dividing the moles of methanol (0.456 mol) by the total moles of the solution (0.456 mol + 10.211 mol = 10.667 mol).
This gives us a mole fraction of:
mole fraction = 0.456 mol / 10.667 mol = 0.0427 or 4.27%.
Learn more about Mole fraction here: https://brainly.com/question/14783710
#SPJ11
the sweetness of honey gradually decreases at a high temperature. also, high-fructose corn syrup (a commercial product in which much of the glucose in corn syrup is converted to fructose) is used for sweetening cold drinks but not hot drinks. what chemical property of fructose could account for both of these observations?
Answers
Answer:
Fructose will cyclize to either furanose or pyranose. Increasing the temperature shifts the equilibrium in the direction of furanose, the less sweet form.
Explanation:
The higher the temperature the less sweet since there will be more furanose than pyranose.
What is the molality of a solution that has 6 mol of CaCl2 in 3 kg of water?
A. 2 m
B. 0.5 m
C. 6 m
D. 3 m
Answers
Answer:
2m
Explanation:
Hope this helps :)
Answer:
Answer is B.
Explanation:
3mol/6kg = 0.5m
N2 + 3H2 to 2NH3 In a certain reaction you start with 4 moles of nitrogen and 10 moles of hydrogen. how many miles of the excess reactant will be left over
Answers
Answer:
Explanation:
We know that for every mole of nitrogen consumed, 3 moles of hydrogen are consumed.
For the nitrogen, this means the reaction can occur 4/1 = 4 times.For the hydrogen, this means the reaction can occur 10/3 = 3.33 times.So, hydrogen is the limiting reactant, and if 10 moles of hydrogen are used, then the reaction will occur 3.33 times.
Hence, this means there is enough nitrogen left for the reaction to occur another 0.67 times, and hence 0.67 moles of nitrogen will be left over.
when you flex your biceps, you are calling on the somatic sensory portion of the nervous system.
Answers
The given statement "When you flex your biceps, you are calling on the somatic sensory portion of the nervous system" is false because the somatic sensory portion of the nervous system is responsible for the detection of sensory information such as touch, pressure, temperature, and pain.
The somatic nervous system, which is responsible for voluntary movement, is made up of two parts: the somatic motor system and the autonomic nervous system. The somatic motor system controls skeletal muscles and is responsible for voluntary movements, such as flexing the biceps. The autonomic nervous system is responsible for involuntary functions such as heart rate, digestion, and breathing.
When you flex your biceps, you are activating the somatic motor system, specifically the motor neurons that control the muscles in your arm. The motor neurons send signals from the brain to the muscles, causing them to contract and produce movement.
Overall, while the somatic sensory portion of the nervous system is involved in sensing stimuli from the environment and transmitting that information to the brain, it is not directly involved in the voluntary movement of muscles.
The question was Incomplete, Find the full content below :
When you flex your biceps, you are calling on the somatic sensory portion of the nervous system. - True or False
Know more about the Nervous system here :
https://brainly.com/question/30299740
#SPJ11