the scientefic methos is founded on
Answers
Answer:
Francis Bacon was the first to formalize the concept of a true scientific method, but he didn't do so in a vacuum. The work of Nicolaus Copernicus (1473-1543) and Galileo Galilei (1564-1642) influenced Bacon tremendously.
Related Questions
How to determine the concentration of hydroxide ion in a saturated solution of zinc hydroxide?
Answers
The concentration of hydroxide ion in a saturated solution of zinc hydroxide can be determined through a series of steps.
To determine the concentration of hydroxide ion in a saturated solution of zinc hydroxide:
1. Calculate the molar solubility of zinc hydroxide (Zn(OH)2) using the solubility product constant (Ksp) value. This can be done by setting up an equilibrium expression and solving for the concentration of hydroxide ion (OH-) in terms of x (molar solubility of Zn(OH)2).
2. Convert the molar solubility of zinc hydroxide to the concentration of hydroxide ion by considering the stoichiometry of the balanced chemical equation. Since zinc hydroxide dissociates into one zinc cation (Zn2+) and two hydroxide ions (OH-), the concentration of hydroxide ion will be twice the molar solubility of zinc hydroxide.
3. Finally, substitute the molar solubility of zinc hydroxide into the expression obtained in step 2 to calculate the concentration of hydroxide ion in the saturated solution of zinc hydroxide.
In summary, to determine the concentration of hydroxide ion in a saturated solution of zinc hydroxide, calculate the molar solubility of zinc hydroxide using the solubility product constant, convert the molar solubility to the concentration of hydroxide ion, and substitute the molar solubility into the expression to obtain the concentration.
Learn more about zinc hydroxide
brainly.com/question/28232373
#SPJ11
Reactions of Benzene
By far the most characteristic reaction of aromatic compounds is substitution at a ring carbon.
•This reaction is called ___________________
Some groups that can be introduced directly on the ring are the halogens, the nitro (–NO2) group, and the sulfonic acid (–SO3H) group.
Halogenation:
Answers
By far the most characteristic reaction of aromatic compounds is substitution at a ring carbon. This reaction is called electrophilic aromatic substitution.
In electrophilic aromatic substitution, an electrophile (a species that is electron-deficient and seeks electrons) attacks the aromatic ring, replacing one of the hydrogen atoms. The electrophile becomes bonded to the ring, and the hydrogen is lost as a proton. This reaction is initiated by a catalyst, usually a Lewis acid, that helps to generate the electrophile.
One common example of electrophilic aromatic substitution is halogenation, where a halogen (e.g., chlorine, bromine) is introduced onto the benzene ring. This reaction proceeds via the formation of a halonium ion intermediate, which is then attacked by the aromatic ring. The final product is a halogen-substituted benzene molecule.
Another example of electrophilic aromatic substitution is nitration, where a nitro group (–NO2) is introduced onto the benzene ring. This reaction proceeds via the formation of a nitronium ion intermediate, which is then attac
Lastly, sulfonation is another example of electrophilic aromatic substitution. In this reaction, a sulfonic acid group (–SO3H) is introduced onto the benzene ring, and this proceeds via the formation of a sulfur trioxide intermediate, which is then attacked by the aromatic ring.
Overall, electrophilic aromatic substitution is a crucial reaction in organic chemistry, and it allows for the synthesis of a vast array of substituted aromatic compounds.
Learn more about electrophilic here:
https://brainly.com/question/31182532
#SPJ11
a car is running with the velocity of 12 kilometre per hour what will be its velocity after 5 seconds if its acceleration is 2 metre
Answers
The velocity after 5 second =19 m/s
Further explanationGiven
Initial velocity =vi = 12 km/h=3.3 m/s
Time=t = 5 s
Acceleration=a = 2 m/s²
Required
Final velocity, vf
Solution
Motion with constant acceleration
Can be formulated :
vf=vi+at
Input the value :
vf=3.3+2.5
vf=9+10
vf=19 m/s
Need help with question 3!
*please answer correctly*
A student increases the temperature of a 300cm3 balloon from 40°C to 110°C.
What will the new volume of the balloon be? Round your answer to one decimal point.
(To convert Celsius to kelvin, add 273.15)
Thank you!!!

Answers
Answer:
\(V_2=367.06\ cm^3\)
Explanation:
Charles law states that volume of the gas is directly proportional to the temperature i.e.
\(\dfrac{V_1}{T_1}=\dfrac{V_2}{T_2}\)
We ave,
V₁ = 300 cm³
T₁ = 40°C = 40 + 273.15 = 313.15 K
T₂ = 110°C = 110 + 273.15 = 383.15 K
Let V₂ be the new volume.
\(V_2=\dfrac{V_1T_2}{T_1}\\\\V_2=\dfrac{300\times 383.15 }{313.15}\\\\V_2=367.06\ cm^3\)
So, the new volume of the balloon is \(367.06\ cm^3\).
How many moles of potassium oxide (K₂O) will be formed when 3.13 moles of K reacts with O₂ according to the following reaction:
4K + O₂ → 2 K₂O
Answers
1.565 moles of K₂O will be formed when 3.13 moles of K reacts with O₂.
What are moles?A mole is a unit of measurement used in chemistry to express the amount of a substance. It is defined as the amount of a substance that contains the same number of particles (atoms, molecules, or ions) as there are in 12 grams of carbon-12. One mole of a substance contains Avogadro's number of particles, which is approximately 6.022 x 10^23.
What is stoichiometric coefficient?Stoichiometric coefficient is the number that appears in front of a chemical formula in a balanced chemical equation. It represents the relative number of moles of that substance that participate in the chemical reaction.
Equation:The balanced chemical equation for the reaction is:
4K + O₂ → 2K₂O
This equation shows that 4 moles of K reacts with 1 mole of O₂ to produce 2 moles of K₂O.
To determine how many moles of K₂O will be formed when 3.13 moles of K reacts with O₂, we need to use stoichiometry.
Assuming there is enough O₂ available for the reaction, we can use the mole ratio from the balanced equation to calculate the amount of K₂O produced.
From the balanced equation, the mole ratio of K to K₂O is 4:2 or 2:1. This means that for every 2 moles of K₂O produced, 4 moles of K are consumed.
Therefore, if 3.13 moles of K are reacted, the amount of K₂O produced will be:
(3.13 mol K) x (2 mol K₂O / 4 mol K) = 1.565 mol K₂O
Therefore, 1.565 moles of K₂O will be formed when 3.13 moles of K reacts with O₂.
To know more about moles, click here
https://brainly.com/question/20486415
#SPJ1
Explain one way the water cycle affects climate. Use complete sentences.
Answers
Answer:
The water cycle will affect the climate by causing rain and snow.
Explanation:
if tempatures increase more rain fall will occur because more water evaporates in the air.
Answer:
That fresh water hydrates the carbon-based lifeforms on earth. But through evaporation, and transpiration from plants leaf surfaces, it gets back into the atmosphere to feed clouds for the next rainfall. The cycling of liquid water and returning vapor creates our climate and our living environment.
if you have a 97.4 gram sample of magnesium hydroxide how many grams of metal do you have?
Answers
The grams of the magnesium metal present is 40.56 g.
What is the mass of the metal?Let us recall that the magnesium hydroxide is composed of two ions and these are the magnesium ions and the hydroxide ions.
Looking at the ions that we have we now would be able to find the number of moles of the compound that we have from the formula;
Number of moles = mass/molar mass
Number of moles = 97.8 g/58 g/mol
= 1.69 moles
The amount of the magnesium ions here is 1.69 moles
Mass of the metal = 1.69 moles * 24 g/mol
= 40.56 g
Learn more about magnesium hydroxide:https://brainly.com/question/24703629
#SPJ1
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
HELP POGGERS!!! I NEED HELP!!!!!!!!!! THIS IS NEEDED IN 10 MINS

Answers
Answer:
(3)
Explanation:
Guaranteed. Look at my comment for linked picture explanation!
What law states that matter cannot be created nor
destroyed, even in a chemical reaction?
A
Newton's Laws of Motion
B
Law of Conservation of Mass
Answers
Answer:
I believe it would be Law of conservation of mass (sorry if I'm incorrect)
Ca(OH)2(s) ? Ca^2+(aq) + 2OH^-(aq) Predict the expected shift, if any, caused by adding the various ions (Ca2+, Na+, Ag+, H+, OH-, NO3-) to a saturated calcium hydroxide solution? Answers and explanations would be greatly appreciated!
Answers
The required correct answer for this the addition of various ions (Ca2+, Na+, Ag+, H+, OH-, NO3-) to a saturated calcium hydroxide solution will cause a shift to the right or left in the equilibrium position, depending on the ion added.
Explanation: The solubility product of Ca(OH)2 is 5.5 x 10^-6.The equation for the dissociation of calcium hydroxide is given below:Ca(OH)2(s) ? Ca2+(aq) + 2OH-(aq)It can be observed from the equation that two hydroxide ions are produced for each calcium ion; thus, the concentration of OH- is twice that of Ca2+.Therefore, the solubility of calcium hydroxide is mainly determined by the concentration of OH- ions in the solution.The addition of the following ions to the solution would cause the following shifts, according to Le Chatelier's Principle:Ca2+: There will be no shift because Ca2+ is already present in the solution and increasing its concentration will not cause any change.Na+: No shift will occur because Na+ is a spectator ion that does not participate in the reaction.Ag+: Ag+ forms a complex ion with OH-, and the equilibrium will shift to the left as a result.H+: The concentration of OH- will decrease as a result of the addition of H+ ions, and the equilibrium will shift to the right to re-establish the equilibrium.NO3-: It has no effect because it is not present in the reaction. OH-: The equilibrium will shift to the left if OH- ions are added to the solution because the concentration of OH- ions is already at its maximum. Hence, the addition of various ions (Ca2+, Na+, Ag+, H+, OH-, NO3-) to a saturated calcium hydroxide solution will cause a shift to the right or left in the equilibrium position, depending on the ion added.
Learn more about expected shift by adding various ions click here https://brainly.in/question/20871293
#SPJ11
Write a balanced equation for the following word equation:nitrogen dioxide + water + oxygen arrow nitric acid
Answers
Answer:
2H2O+4NO2+O2=4HNO3
Explanation:
LEFT HAND SIDE(L.H.S) RIGHT HAND SIDE(R.H.S)
N=1X10X2 =20 N=1X10X2=20
H=2X5X2 =20 H=1X10X2=20
O=1X5X2+10X2X2+2 X5 O=3X10X2=60
=60
First multiply H2O by 2 to get an even no. of oxygen on L.H.S
make a similar chart at every turn to record the changes in number.
then multiply R.H.S by 2 to equate the hydrogen
multiply N by 2 on LHS to equate them.
the no of O changes
Multiply RHS by 2 again,
equate the LHS by multiplying NO2 by 2 again, i.e total 4
you now have the answer
The law that relates the temperature and volume of a gas to each other is known as.
Answers
Type the correct answer in each box.
Which chemical symbols will complete the equation for this single displacement reaction?
2NaBr + Cl2 → 2 __ __ + __ 2
Answers
Answer:
2Naci+Br2
Explanation:
i hope it is help full
Answer:
Na//Cl//Br
Explanation:
1st Define Covalent bond and it's type
2nd What would be the electron dot structure of carbon dioxide which has the formula CO2.
3rd what would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Answers
Answer:
COVELENT BOND:-The chemical bond formed by the sharing of an electron pair between two atoms so that both the atoms get their octet complete is called covalent bondSINGLE COVELENT BOND:-it is formed by sharing of one pair of electron between two atomsDOUBLE COVELENT BOND:-it is formed by sharing of two pair of electron between two atomsTRIPLE COVELENT BOND:- it us formed by sharing of three pair of electron beyween two atoms2)Electron dot structures of carbon dioxide:-Oxygen atom contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Carbon atom contains four valence electrons, resulting in zero lone pairs. Therefore, it is doubly bonded to each oxygen atom.
3))Sulfur has an atomic number is 16 with the
symbol as 'S'
The electronic configuration of sulfur is said to be 2,8,6The valence electrons present in sulfur is 6.Sulfur forms an octet structure by connecting 8 sulfur atoms with each other by the formation of single covalent bonds.The sulfur molecule's chemical formula is S8.Sulfur is usually used in the manufacture of sulphuric acid.Explanation:
HOPE IT HELPS YOU #ITZADMIRER

A covalent bond is a type of bond that is typically characterized by sharing of electrons between the atoms of a non-metallic chemical element.
What is a covalent bond?
A covalent bond can be defined as a type of bond that exists in chemical compounds, and it is typically characterized by sharing of electrons between the atoms of a non-metallic chemical element.
The types of covalent bond.In Chemistry, there are two (2) main types of covalent bond and these include:
Polar covalent bondNon-polar covalent bondThe carbon atoms of carbon dioxide (\(CO_2\)) has four valence electrons and the oxygen atoms has six valence electrons. Thus, electron dot structure of carbon dioxide represents a double covalent bond.
Read more on covalent bond here: https://brainly.com/question/20602843
Use the drop-down menus to identify the class of compounds to which each structure belongs. A skeletal chain goes up, down, up, down to C double bonded to O, up to O H. A skeletal chain goes up to O, down, up, down, up. A hexagonal ring is single bonded to C l on one corner. N has a pair of electron dots above, and is single bonded to R Superscript 1, wedge bonded to H, and wavy bonded to H.
Answers
Functional group can be interconverted into each ther by the means of suitable reagent. Functional group are responsible for the chemical and physical properties of a compound.
What is functional group?A group of atoms forming a component of a molecule that accounts for a particular function or chemical behavior is called a functional group. There are so many functional group in organic chemistry.
a. A skeletal chain goes up, down, up, down to C double bonded to O, up to O H is Carboxylic group COOH.
b. A skeletal chain goes up to O, down, up, down, up. A hexagonal ring is single bonded to C l on one corner. this belongs to cyclic substituted compound.
c. N has a pair of electron dots above, and is single bonded to R Superscript 1, wedge bonded to H, and wavy bonded to H. This belongs to primary amine compound.
To know more about functional group, here:
https://brainly.com/question/14618322
#SPJ1
All of the molecules in a cup of water are moving at the same speed or at a variety of speeds explain ?
Answers
Answer:
They will move at the same speed
Explanation:
This will depend on the heat of the water. The hotter the water is the more the molecules move around. As the water cools it will make the molecules move slower.
Hope this helps! :)
Does Jarren love Maria more or does Maria love Jarren more?
(Not for school)
Answers
Answer:
Maria
Explanation:
Answer:
Maria loves Jarren more is the answer.
Helped by QueenTlove Have an nice day
Which substance is an electrolyte? insulin cortisol potassium epinephrine glucose
Answers
The substance that is an electrolyte is: potassium
In chemistry an electrolyte is known as a substance or compound that has the capacity of being an electrical conductor when it is dissolved into a solution with water. Some examples of electrolytes are: potassium, common salt (NaCl), calcium.
What is a solution?
In chemistry a solution is known as a homogeneous mixture of two or more components called:
Solvent: it usually is in a major amount than the soluteSolute: it usually is in less amount than the solvent
Learn more about chemical solution at: brainly.com/question/13182946
#SPJ4

6. A family decided to take a trip to Europe. The plane they took flew at an average speed of 500 miles per hour
during the flight. The plane covered a distance of 5,000 miles during the flight. How long was the flight?
Your answer
Answers
Answer:
10 hours
Explanation:
Answer:
10
Explanation:
Where does the storm come from in places like Taiwan, North Carolina, Galveston etc.
Answers
Taiwan experience Typhoons, North Carolina Hurricanes and thunderstorm, and Galveston Hurricanes
Taiwan does not experience tornadoes, which are common on the mainland. However, from the end of summer to the beginning of autumn, it is directly hit by typhoons (tropical cyclones), some of the strongest in the world. Typhoons in Taiwan can cause severe damage to crops and sometimes severe flooding. With an annual rainfall of 2,600 mm, Taiwan is one of the wettest places in the world and is often brought to the island by seasonal typhoons.
North Carolina is one of the most hurricane-prone states. With its coastline jutting into the Atlantic Ocean, the state is like a catcher's mitt for northbound storms. And the threat of hurricanes continues to grow as climate change changes the environment.
Towering cumulonimbus clouds and thunder are not uncommon on summer afternoons and evenings in North Carolina. During the warmer months of the year, the state's weather is determined by local-scale convective processes as the jet stream retreats northward. Thunderstorms in North Carolina bring strong winds and heavy rains, which can cause localized flash floods. Sometimes these storms produce hail, tornadoes, or damage straight winds.
Also known as the Galveston Hurricane of 1900, Great Galveston Hurricane, and Hurricane of September 1900 (tropical storm), it is one of the deadliest natural disasters in U.S. history, claiming more than 8,000 lives. When the storm hit the island city of Galveston, Texas, it was a Category 4 hurricane, his second strongest designation on the Suffer-Simpson Hurricane Scale.
To know more about Atlantic Ocean
https://brainly.com/question/543870
#SPJ1
Hello please help me with this question
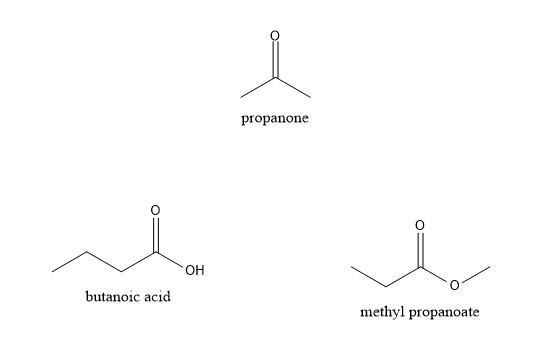
1. Draw the following organic compounds:
a. Propanone
b. Butanoic acid
c. Methyl Propanoate
Answers
Propanone is a ketone with skelton CH₃COCH₃. Butanoic acid is a 4 carbon carboxylic acid and methyl propanoate is an ester as given un the image.
What are ketones ?Ketones are organic compounds having the functional group of CO. Propanone is a ketone with three carbons including the CO group. Carboxylic acids are compounds containing COOH group.
Butanoic acid is a carboxylic acid with 4 carbon atoms including the carbon atom in the COOH group. Carboxylic acids are different in properties from the inorganic acids.
Esters are formed by the reaction of acids with alcohol with the functional group COOR, where R can be any alkyl group. Methyl propanoate is an ester with 4 carbon atoms.
Find more on organic compounds:
https://brainly.com/question/5994723
#SPJ1
(e) as the reaction occurs at constant temperature, does the pressure inside the container increase, decrease, or remain the same? explain.(e) as the reaction occurs at constant temperature, does the pressure inside the container increase, decrease, or remain the same? explain.
Answers
This can be explained by the ideal gas law, which states that pressure is proportional to the number of gas molecules within a container at a constant temperature. Therefore, as more gas molecules are produced during the reaction, the pressure within the container will increase.
What is Gas Law?
Gas laws are a set of fundamental principles that describe the behavior of gases under different conditions of temperature, pressure, and volume. The most commonly studied gas laws are Boyle's law, Charles's law, Gay-Lussac's law, and the combined gas law. Boyle's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. Charles's law states that at a constant pressure, the volume of a gas is directly proportional to its temperature.
Gay-Lussac's law states that at a constant volume, the pressure of a gas is directly proportional to its temperature. The combined gas law combines these three laws to relate pressure, volume, and temperature of a gas. Gas laws are important in understanding the behavior of gases in many real-world applications, such as in chemical reactions, combustion engines, and weather forecasting.
The pressure inside the container will increase as the reaction occurs. This is because the reaction between magnesium and hydrochloric acid produces hydrogen gas, which is a gas that occupies space within the container. As the amount of gas within the container increases, the pressure inside the container will also increase if the temperature is held constant.
Learn more about Gas Law from given link
https://brainly.com/question/27870704
#SPJ1
A 500 mL flask is filled with krypton (Kr) at STP. How many moles of Kr are present?
Answers
The number of moles present in a 500mL flask that is filled with krypton (Kr) at STP is 0.022 moles.
How to calculate number of moles?The number of moles in a substance can be calculated using the following ideal gas law equation as follows:
PV = nRT
Where;
P = pressureV = volumeT = temperaturen = no of molesR = gas law constantAccording to this question, a 500mL flask is filled with krypton (Kr) at STP. The values of T, P and R at STP are as follows:
T = 273KP = 1 atmR = 0.0821 Latm/K1 × 0.5 = n × 0.0821 × 273
0.5 = 22.41n
n = 0.022 moles
Learn more about moles at: https://brainly.com/question/21050624
#SPJ1
what is the molar concentration of a solution formed by dissolving 450.0 mg of nacl to make 100.0 ml of solution? molar mass of nacl is 58.44 g/mol.
Answers
The molar concentration of the NaCl solution formed by dissolving 450.0 mg of NaCl in 100.0 mL of solution is 0.0770 mol/L.
To determine the molar concentration of a solution formed by dissolving 450.0 mg of NaCl (sodium chloride) in 100.0 mL of solution, we need to convert the mass of NaCl to moles and then calculate the molarity (mol/L).
First, we convert the mass of NaCl to grams:
Mass of NaCl = 450.0 mg = 450.0 mg × (1 g/1000 mg) = 0.450 g
Next, we calculate the number of moles of NaCl using its molar mass:
Molar mass of NaCl = 58.44 g/mol
Number of moles of NaCl = mass of NaCl / molar mass of NaCl
Number of moles of NaCl = 0.450 g / 58.44 g/mol = 0.00770 mol
Now, we can determine the molar concentration (Molarity) of the solution using the formula:
Molarity (M) = Number of moles / Volume of solution in liters
Volume of solution = 100.0 mL = 100.0 mL × (1 L/1000 mL) = 0.100 L
Molarity (M) = 0.00770 mol / 0.100 L = 0.0770 mol/L
Therefore, the molar concentration of the NaCl solution formed by dissolving 450.0 mg of NaCl in 100.0 mL of solution is 0.0770 mol/L.
Learn more about molar concentration from below link
https://brainly.com/question/26255204
#SPJ11
1. What is the molarity of a Mg(OH)2 solution if 30 mL is required to neutralize 85 mL
of a 2.0 M solution of HNO3?
Answers
Answer:
A solution is a homogeneous mixture of two or more chemical substances. If we have a solution
made from a solid and a liquid, we say that the solid is dissolved in the liquid and we call the
solid the solute and the liquid the solvent. Initially, we will consider only solutions of a solid in
water. If a solution has a small amount of solute in a large amount of solvent, we say that the
solution is dilute (or that we have a dilute solution). If a solution has a large amount of solute
for a certain amount of solvent, we say that the solution is concentrated (or that we have a
concentrated solution). We see that the terms dilute and concentrated are not precise and are
merely used to give a rough indication of the amount of solute for a given amount of solvent.
The amount of solute in a given amount of solvent (or solution) is called the concentration of
the solution. In this course, we will consider two ways of expressing concentration - mass
percent and molarity. We will consider molarity here and mass percent later.
Explanation:
Calculate the pH when 25.0ml of 0.90M CH3COOH is mixed with 25.0ml of 0.45M NaOH.
Answers
pH of mixed solution = 4.3
Further explanationA buffer solution is a solution that can maintain a good pH value due to the addition of a little acid or a little base or dilution.
The buffer solution can be acidic or basic
Acid buffer solution consists of weak acids and their salts.
\(\tt \displaystyle [H^+]=Ka\times\frac{mole\:weak\:acid}{mole\:salt\times valence}\)
valence according to the amount of salt anion
mol CH₃COOH = 25 ml x 0.9 = 22.5 mlmol
mol NaOH = 25 ml x 0.45 = 11.25 mlmol
ICE method :
Reaction
NaOH + CH₃COOH ---> CH₃COONa + H₂O
11.25 22.5
11.25 11.25 11.25 11.25
11.25 11.25 11.25
mol acid = 11.25, mol salt = 11.25, Ka CH₃COOH = 5.10⁻⁵
Then [H⁺]=
\(\tt [H^+]=5.10^{-5}.\dfrac{11.25}{11.25\times 1}\rightarrow salt~valence=1\\\\(H^+]=5.10^{-5}\\\\pH=5-log5=4.3\)
If 400cm³ of Q was collected at 250°c and 1.20×10³nm², calculate the volume it would occupy at STP
Answers
Answer:
2.481cm³
Explanation:
this is general has equation so the formula is
P1V1/T1 = P2V2/T2
At STP, pressure is 1.01*10⁵ and temperature is 273K
((1.20*10³)400)/(250+273) = ((1.01*10⁵)V2/(273)
V2 = 2.481cm³
I need help with this please

Answers
Answer:
raising the Concentration of liquid
raise the temperature of the system
bubbles of hydrogen gas
Draw Lewis structure(s) showing all possible equivalent resonance forms for the nitryl chloride molecule (NO2Cl Draw one structure per sketcher box, and separate any added sketcher boxes with the symbol. Do NOT show any n charges in your drawings. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule.
Answers
The nitryl chloride molecule (NO₂Cl) consists of a central nitrogen atom bonded to two oxygen atoms and a chlorine atom, with the double bond delocalized between one oxygen and the nitrogen atom in resonance. The Lewis structure is given below.
The nitryl chloride molecule (NO₂Cl) can exhibit resonance, resulting in multiple equivalent resonance structures. Here are the possible resonance forms for NO₂Cl:
Resonance Form 1:
Cl
|
O = N - O
Resonance Form 2:
Cl
|
O - N = O
These two resonance structures represent the different arrangements of the double bond between the nitrogen and oxygen atoms. The actual structure of nitryl chloride is a hybrid of these resonance forms, with the double bond delocalized or spread out over both nitrogen and oxygen atoms.
learn more about Lewis structure here:
https://brainly.com/question/20300458
#SPJ4