The+percent+by+mass+of+sodium+chloride+in+a+solution+of+14.0+g+of+sodium+chloride+in+enough+water+to+make+91.0+g+of+solution+is:_________
a. 15.4%
b. 84.6%
c. 13.3%
d. 86.7%
e. 115%
Answers
The correct answer is [a].The percent by mass of sodium chloride in a solution of 14.0 g of sodium chloride in enough water to make 91.0 g of solution is 15.4%.
What is a solution?Solution, in chemistry, homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility.
The term solution is commonly applied to liquid state of matter, but solutions of gases and solids are possible. Air, for example, is a solution consisting chiefly of oxygen and nitrogen with the trace amounts of several other gases, and brass is a solution composed of copper and zinc.
Solutions play a significant role in the processes of life. In blood plasma, oxygen from the lungs dissolves and chemically joins with hemoglobin in red blood cells before being delivered to the body's tissues. The byproducts of digestion are also transported to other bodily areas in solution. Many real-world situations can be solved by using liquids' capacity to dissolve other liquids or solids.
Chemists take advantage of differences in the solubility to separate and purify materials and to carry out chemical analysis. Most chemical reactions occur in solution and are influenced by solubilities of the reagents. Materials for chemical manufacturing equipment are selected to resist solvent action of their contents.
Percent by mass= \(\frac{weight of solute}{weight of solution}\)*100= \(\frac{14}{91}*100= 15.4%\)\(%\)%
To know more about sodium chloride visit: brainly.com/question/9811771
#SPJ4
Related Questions
24. what is the most likely method of decay of the radioactive isotope technicium-99 (99tc)? a. alpha decay b. beta decay c. electron capture d. positron emission e. both electron capture and positron emission
Answers
The radioactive isotope Technium-99 decays most likely through alpha decay (99tc). An atomic nucleus emits an alpha particle during the radioactive decay process known as "alpha decay".
and then changes or "decays" into a separate atomic nucleus with a mass number that is decreased by four and an atomic number that is decreased by two. The nucleus of an atom of helium-4 is the same as an alpha particle. Radioisotopes are an element's radioactive isotopes. They are the atoms with unstable neutron-proton combinations or excess energy in their nuclei. During those processes, the radionuclide is said to experience radioactive decay, albeit the surplus energy may be put to use in any number of ways.
Learn more about radioactive isotope here
https://brainly.com/question/1907960
#SPJ4
A radiosotope emits a positron to form titanium -48. Express your answer as a nuclear equation.
Answers
The nuclear equation for the formation of titanium-48 from a radiosotope emitting a positron is: Radiosotope → Titanium-48 + Positron.
To express the formation of titanium-48 from a radiosotope emitting a positron, we can write the nuclear equation as follows:
Reactant Nucleus: Radiosotope
Product Nucleus: Titanium-48
Particle Emitted: Positron
The nuclear equation can be written as:
Radiosotope → Titanium-48 + Positron
This equation represents the decay of the radiosotope, where it emits a positron and forms titanium-48 as the product nucleus.
Learn more:About radiosotope here:
https://brainly.com/question/11913067
#SPJ11
What is the empirical formula of a compound that is 70.62% Hg, 12.48%
Cl, and 16.9% O?
Answers
Answer:
Empirical Formula ClHgO3 Mercurous Chlorate
Explanation:
Cl=12.48% Hg=70.62% O=16.9%
A student lists three temperature measurements: 100°F, 100°C, and 100 K. Of the three measurements, 100 K is the highest temperature.a. Trueb. False
Answers
Answer:
The answer is False.
Explanation:
To find which is the highest temperature we need to convert the °C and °F units to °C:
\( T_{1} = 100 K \)
\( T_{2} = 100 ^{\circ} C + 273 = 373 K \)
\( T_{3} = \frac{5}{9}*(100 ^{\circ} F - 32) = 37.78 ^{\circ} C \)
\( T_{3} = 37.78 ^{\circ} C + 273 = 310.78 K \)
Therefore, the answer is False, since the highest temperature is 100 °C.
I hope it helps you!
Answer:
The answer is False, or ¨F¨.
You dissolve 0.66 g of potassium chloride (KCl) in 700 ml of water.What is the molarity of the solution?(From the periodic table: 1 mol K = 39.10 g; 1 mol Cl = 35.453 g)=Enter the value rounded to three decimal places with no units
Answers
Answer
0.009 mol/L
Explanation
Given:
Mass of KCl = 0.66 g
Volume of water = 700 mL = 0.7 L
From the periodic table: 1 mol K = 39.10 g; 1 mol Cl = 35.453 g
What to find:
The molarity of the solution
Step-by-step solution:
The formula to calculate molarity is:
\(\text{Molarity }=\frac{Mole}{Volume\text{ in L}}\)The first step is to calculate the molar mass of KCl
KCl = Mass of 1 mol K + Mass of 1 mol Cl
KCl = 39.10 g + 35.453 g
KCl = 74.553 g
So the molar mass of KCl = 74.553 g/mol
The next step is to determine the number of moles of KCl in 0.66g of KCl:
\(\text{Mole }=\frac{Mass}{Molar\text{ mass}}=\frac{0.66\text{ g}}{74.553\text{ g/mol}}=8.852762464\times10^{-3}mol\)Put the values of mole and volume into the molarity formula above to determine the molarity of the solution:
\(\begin{gathered} \text{Molarity }=\frac{8.852762464\times10^{-3}\text{ mol}}{0.7\text{ L}}=0.00885276molL^{-1} \\ To\text{ thr}ee\text{ decimal places,} \\ \text{Molarity }=0.009\text{ }molL^{-1} \end{gathered}\)The molarity is 0.009 mol/L
HELP ME PLEASE WILL GIVE BRAINLY NO BAD ANSWERS

Answers
Answer:
7.17
Explanation:
convert 5.50g to moles of salicylic acid. Then use mole ratios and convert the moles of salicylic acid to moles of Asprin(mole ratio is 1:1). Then convert the moles to grams using the molar mass of asprin.
What does Seltzer Water have in common with Limestone ?
Answers
describe the three states of matter that are present when snow melts
Answers
Answer:
solid liquid and gas
Explanation:
ice is a solid then it melts to liquid and evaporates into gas
Answer:
Snow is basically water;
ice/snow is solid state
water is liquid state
vapour is gaseous state
Explanation:
Emma has 37 green apples and 15 red apples. What is the ratio of red apples to green apples?
Answers
Answer:
15:37
Explanation:
cant simplify because 37 is prime
The chemical equation below represents an unbalanced chemical reaction:
Fe + 0, → Fe,o,
When the equation is balanced, what coefficient is needed for Fe2O3?
A
1
B
2
С
3
D
4
Answers
Answer:
2
Explanation:
Why does soap work to remove oil? (Note that most oils can be considered non-polar. A soap molecule has one end that is polar and one end that is non-polar.)
Answers
Answer:
Soap breaks up the oil into smaller drops, which can mix with the water.
Explanation:
This works because soap is made up of molecules with two different ends.
The soap molecules works as a surfactant for the water oil interphase. The hydrophobic head of the soap molecules trap the oil and dirt ad remove from the surface.
What are surfactants?Surfactants are chemical substances used to reduce the interfacial tension between substance boundary. Soaps and detergents acts as surfactants which are used to remove the dirt and oil from material surface.
Soap has both polar and non-polar characteristics. The alkaline part in the soap causes it to have a polar head, and because of the electric charge on this head, the soap is hydrophilic. A hydrophilic part of soap is drawn to water and will cling to it.
In this instance, the positive-charged hydrogen atoms of the water's hydrogen atoms match with the polar head of the soap molecule. The other part of soap is a fatty acid tail, which is hydrophobic and can bind with the oil and dirt. The trapped oil molecules are then be removed by water.
To find more on surfactants refer here:
https://brainly.com/question/3062579
#SPJ2
Explain why it is necessary to use a mixture, alumina and cryolite, rather than just
alumina
Answers
Explanation:
The mixture of cryolite and aluminium oxide has a lower melting point than pure aluminium oxide. This means a lower amount of energy is required to establish effective conditions for electrolysis and thus makes it more cost effective.
Concentration of a Drug in the Bloodstream The concentration of a certain drug in a patient's bloodstream thr after injection is given by 0.2t C (t) = +2 +1 mg/cm² Evaluate lim C (t) and interpret your < > result.
Answers
the drug concentration will not stabilize in the patient's bloodstream and will continue to increase indefinitely, which could have adverse effects on the patient.
The given drug concentration formula is C(t) = 0.2t + 2 + 1 mg/cm². To find lim C(t), we need to evaluate the limit as t approaches infinity. As t increases without bound, the 0.2t term dominates the equation, making the other two terms negligible. Therefore, lim C(t) = infinity. This means that the drug concentration in the patient's bloodstream will continue to increase indefinitely, which can be a cause for concern if the drug is not properly metabolized or excreted from the body. It is important for healthcare professionals to monitor drug concentrations in patients to avoid toxicity or adverse effects. To find the limit as t approaches infinity, lim C(t), we can analyze the function. As t increases, the 0.2t term will dominate the constant term, 2. Therefore, the concentration of the drug in the bloodstream will keep increasing without bounds as time goes on. Mathematically, lim (t→∞) C(t) = ∞. This result indicates that the drug concentration will not stabilize in the patient's bloodstream and will continue to increase indefinitely, which could have adverse effects on the patient.
To know more about drug visit:
https://brainly.com/question/29767316
#SPJ11
2-2. (10 points) At the bottom of a flat, quiescent (i.e., no advection) lake there are solid deposits of manganese. Due to a change in redox conditions manganese is dissolving into the water and just above the manganese deposits the concentration is 60μg/L. The lake serves as a water source for the water treatment plant that does not currently have manganese treatment. The water system's goal is for manganese to remain below its detection limit of 2μg/L because manganese accumulation in the distribution system can lead to black water events. a) What is the dominate transport mechanism in the lake? b) The intake at the water treatment plant is 1ft from the lake bottom. How long does the water treatment plant have before it needs to start treating for manganese? Use equation 1−18 in Benjamin and Lawler that is provided for stagnant conditions. The diffusion coefficient for manganese is 6.88×10−6 cm2/s. c) As a temporary solution the water treatment plant plans to raise the water intake level so that it has 1 year to design and install a manganese treatment system. What minimum height above the lake bottom should the intake be raised?
Answers
The dominant transport mechanism in the lake is diffusion. The water treatment plant has a limited time before it needs to start treating for manganese, and the minimum height above the lake bottom for the water intake to provide one year for designing and installing a manganese treatment system needs to be determined.
Dominant transport mechanism: Diffusion is the main transport mechanism in the lake. This means that manganese is gradually diffusing from the solid deposits at the lake bottom into the water column.
Initial concentration: The concentration of manganese just above the deposits is given as 60 μg/L.Detection limit: The water treatment plant aims to keep the manganese concentration below the detection limit of 2 μg/L to prevent black water events.Time to start treating: To determine how long the water treatment plant has before it needs to start treating for manganese, we can use Equation 1-18 in Benjamin and Lawler, which is provided for stagnant conditions. The equation is:t = (L^2) / (4D)
where t is the time in seconds, L is the distance from the bottom (1 ft or 30.48 cm), and D is the diffusion coefficient of manganese (6.88×10^(-6) cm^2/s).
Calculation Plugging in the values into the equation, we can calculate the time it takes for manganese to reach the water intake level.
t = (30.48^2) / (4 × 6.88×10^(-6)) = 126,707 seconds
Converting seconds to days: 126,707 seconds ÷ (24 hours/day × 3600 seconds/hour) ≈ 1.47 days
Therefore, the water treatment plant has approximately 1.47 days before it needs to start treating for manganese.
Minimum intake height: To provide one year for designing and installing a manganese treatment system, the intake should be raised to a height where the time it takes for manganese to reach that level is one year.
t = (L^2) / (4D)
Rearranging the equation to solve for L:
L = √(4Dt)
Plugging in the values: L = √(4 × 6.88×10^(-6) cm^2/s × (1 year × 365 days/year × 24 hours/day × 3600 seconds/hour))
L ≈ 49.65 cm or 0.163 ft
The minimum height above the lake bottom that the intake should be raised to is approximately 0.163 ft.
The dominant transport mechanism in the lake is diffusion, where manganese is slowly diffusing from the solid deposits into the water column. The water treatment plant has approximately 1.47 days before it needs to start treating for manganese to maintain concentrations below the detection limit. To provide one year for designing and installing a treatment system, the intake should be raised to a minimum height of approximately 0.163 ft above the lake bottom.
Learn more about Manganese:
https://brainly.com/question/28533522
#SPJ11
Is Spinach Quiche an Element , Compound , Heterogeneous, or a Homogenous
Answers
Answer:
Homogeneous
Explanation:
PLEASE ANSER QUICK ITS URGENT 40 POINTS
Scuba tanks are often pressurized to about 204 atm before the start of the dive.
What is the pressure of 204 atm in kPa?
[ ? ] kPa
Round your answer to three significant figures.
Pressure (kPa)

Answers
Answer:
1 atm = 101.325 kPa
Therefore, 204 atm = 204 * 101.325 = 20670.3 kPa
Rounded to three significant figures, the pressure of 204 atm is 2.07 x 10^4 kPa.
why would a preheat coil be used on the energy-recovery wheel
Answers
A preheat coil is used on an energy-recovery wheel to address two main reasons: to prevent frost formation and to improve energy efficiency.
Frost formation is a common issue in energy-recovery systems, especially in colder climates or during winter months. When cold outdoor air passes through the energy-recovery wheel, the moisture in the air can condense and freeze on the wheel's surface.
This frost buildup reduces the wheel's effectiveness in transferring heat and can even lead to operational issues.
By incorporating a preheat coil, the incoming outdoor air is warmed up before it reaches the energy-recovery wheel, preventing frost formation and ensuring continuous operation.
The second reason for using a preheat coil is to improve energy efficiency. In some situations, the outdoor air may be too cold for the energy-recovery wheel to effectively transfer heat.
By preheating the outdoor air using a coil, the incoming air temperature is raised to a level that allows for more efficient heat transfer within the wheel.
This enhances the overall energy-recovery process, reducing the workload on the primary heating system and improving the HVAC system's efficiency.
In summary, a preheat coil is used on an energy-recovery wheel to prevent frost formation and improve energy efficiency by raising the temperature of the incoming outdoor air.
This ensures uninterrupted operation, enhances heat transfer, and optimizes the performance of the energy-recovery system, particularly in colder climates or during winter seasons.
Learn more about frost from the given link
https://brainly.com/question/23045790
#SPJ11
express balanced equations for the production of nitric acid from nitrogen
Answers
There are several methods for producing nitric acid from nitrogen, but two common methods are:
1. Ostwald process:
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
2 NO(g) + O2(g) → 2 NO2(g)
3 NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
Overall equation:
4 NH3(g) + 3 O2(g) + H2O(l) → 2 HNO3(aq) + 2 NO(g)
2. Birkeland-Eyde process:
N2(g) + 3 H2(g) → 2 NH3(g)
2 NH3(g) + 3 O2(g) → 2 NO(g) + 3 H2O(g)
2 NO(g) + O2(g) → 2 NO2(g)
3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)
Overall equation:
N2(g) + 3 O2(g) + 3 H2(g) → 2 HNO3(aq)
What elements did the first periodic table stop on?
Answers
Answer:
Bismuth
Explanation:
In 1869 Mendeleev published his first Periodic System ending with Bismuth.
In 1871 he updated his system to end with Uranium.
What is an Isochron map
Answers
Answer:
An isochrone map in geography and urban planning is a map that depicts the area accessible from a point within a certain time threshold. An isochrone is defined as "a line drawn on a map connecting points at which something occurs or arrives at the same time".
Explanation:
Answer:
An isochrone (iso = equal, chrone = time) is defined as "a line drawn on a map connecting points at which something occurs or arrives at the same time". In hydrology and transportation planning isochrone maps are commonly used to depict areas of equal travel time.
Explanation:
BRAINLIEST plz
Deficiency of vitamins in the body is known as
Answers
Deficiency of vitamins in the body is known as Vitamin deficiency anemia
In an ideal situation where no heat energy is produced, what is the relationship between the chemical energy provided by the battery and the electrical energy produced according to the Law of Conservation of Energy?
Answers
Answer:
See explanation
Explanation:
The principle of conservation of energy states that energy can neither be created nor destroyed but can be converted from one form to another. Hence, chemical energy in a battery can be converted to electrical energy.
Usually, the conversion of energy from one form to another is not 100% efficient according to the second law of thermodynamics. Some energy is wasted in the process, sometimes as heat.
Hence, in an ideal situation where no heat energy is produced; all the chemical energy is converted to electrical energy (100% energy conversion). There will be no energy loss if no heat is produced.
Which electron dot diagram shows how hydrogen and oxygen are bonded together in the compound H2O?
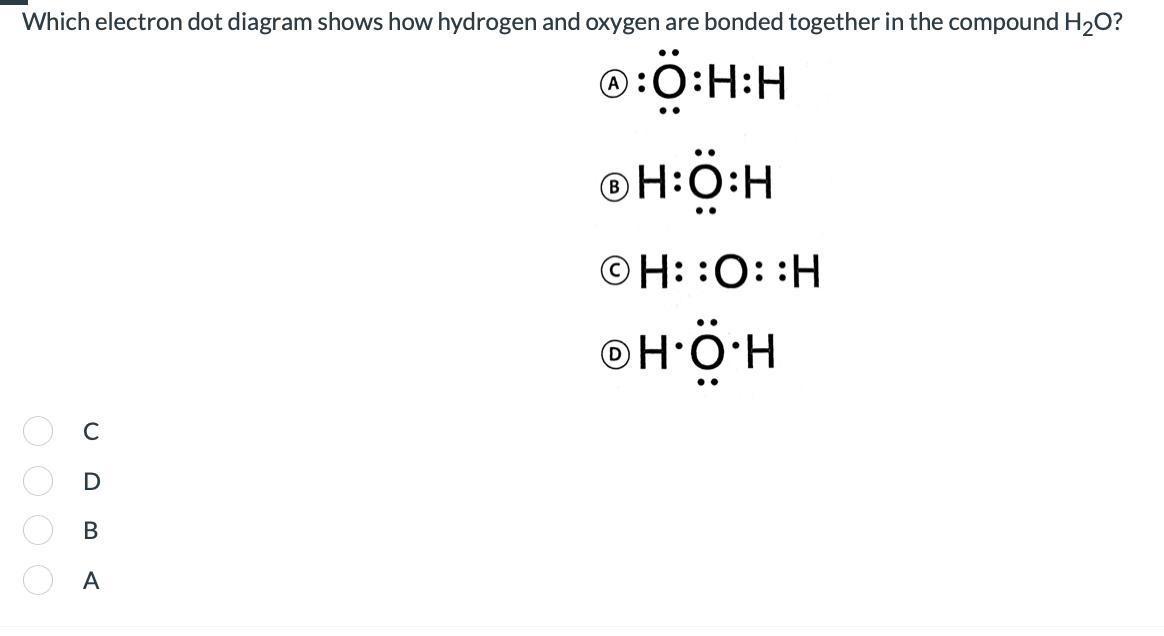
Answers
The correct electron dot structure of water is shown by option B
What is electron dot structure?Each valence electron in the electron dot structure is represented by a dot that is positioned around the element's atomic symbol.
The Lewis dot structure, sometimes referred to as the electron dot structure, uses dots to represent the valence electrons of an atom.
Water contains two hydrogen and one oxygen atom with the oxygen atom having two lone pairs of electrons as shown above in the question.
Learn more about electron dot structure:https://brainly.com/question/9840379
#SPJ1
Mercury(I) ions (Hg2+2) can be removed from solution by precipitation with Cl−. Suppose that a solution contains aqueous Hg2(NO3)2.
Write complete ionic equation to show the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate.
Express your answer as a chemical equation. Identify all of the phases in your answer.
Answers
The phases in the equation are: aq (aqueous) - for Hg2(NO3)2 and NaCl solutions, s (solid) - for Hg2Cl2 precipitate, aq (aqueous) - for NaNO3 solution
The complete ionic equation for the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate is:
Hg2^2+ (aq) + 2Cl^- (aq) → Hg2Cl2 (s)
The reaction involves the precipitation of solid Hg2Cl2 due to the reaction of mercury(I) ions (Hg2^2+) with chloride ions (Cl^-) from the sodium chloride solution. The nitrate ions (NO3^-) from the Hg2(NO3)2 solution combine with the sodium ions (Na+) from the sodium chloride solution to form aqueous sodium nitrate (NaNO3).
The overall chemical equation for this reaction is:
Hg2(NO3)2 (aq) + 2NaCl (aq) → Hg2Cl2 (s) + 2NaNO3 (aq)
To know more about ionic equation visit:
https://brainly.com/question/13887096
#SPJ11
Which elements do not have the same number of atoms on both sides of the equation?
Na and O
H and S
Na and H
S and O

Answers
Answer: Na
Explanation:
on the left it says Na, and on the right it says Na2
If 10 moles of P4S3 was used, how many grams of P4O6 was produced? Leave up to 3 decimal places when possible.
Answers
If 10 moles of P4S3 were used, the mass of P4O6 produced would be 2838.8 grams.
To determine the number of grams of P4O6 produced from 10 moles of P4S3, we need to use the balanced chemical equation and the molar masses of the compounds involved.The balanced equation for the reaction between P4S3 and oxygen to produce P4O6 is:
P4S3 + 8 O2 → P4O6 + 6 SO2
From the balanced equation, we can see that the molar ratio between P4S3 and P4O6 is 1:1. This means that for every 1 mole of P4S3 consumed, 1 mole of P4O6 is produced.The molar mass of P4S3 is 220.25 g/mol, and the molar mass of P4O6 is 283.88 g/mol.
To calculate the mass of P4O6 produced, we can use the following equation:
Mass of P4O6 = Moles of P4O6 × Molar mass of P4O6
Since the molar ratio between P4S3 and P4O6 is 1:1, the number of moles of P4O6 produced is also 10 moles.
Mass of P4O6 = 10 moles × 283.88 g/mol = 2838.8 grams
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
Cuáles son los efectos de la temperatura, la presión y el volumen en los cambios de estado de la materia. *???
Answers
Soryyyyyy I needed pointsss
what is hydraulic pressure?
Answers
inorganic chemicals can exist only as the elemental form shown in the periodic table. True or False
Answers
The given statement "inorganic chemicals can exist only as the elemental form shown in the periodic table". is False. Inorganic chemicals can exist in various forms, including as compounds with other elements.
The periodic table lists all known elements, including inorganic elements, but it does not imply that inorganic chemicals exist only as elemental forms. Inorganic compounds can be made up of two or more elements, including metals and nonmetals, and can be formed through various chemical reactions.
Examples of inorganic compounds include water (H₂O), sodium chloride (NaCl), and carbon dioxide (CO₂), among many others. Inorganic chemistry is the study of these compounds, their properties, and their reactions.
To know more about inorganic chemicals refer here:
https://brainly.com/question/28786378#
#SPJ11
Help I’m so confused

Answers
Answer:
Me too. What is this for? A Lab. You are missing some kind of key info bud.
Explanation: