transition metals, located in the center of the periodic table, have many essential uses as elements and form many important compounds as well. calculate the molecular mass of the following transition metal compound: [pt(nh3)4brcl]cl2
Answers
The molecular mass of the transition metal compound [Pt(NH3)4BrCl]Cl2 is 449.49 amu.
To calculate the molecular mass of the transition metal compound [Pt(NH3)4BrCl]Cl2, follow these steps:
1. Determine the atomic masses of the individual elements in the compound.
- Pt (platinum): 195.08 amu
- N (nitrogen): 14.01 amu
- H (hydrogen): 1.01 amu
- Br (bromine): 79.90 amu
- Cl (chlorine): 35.45 amu
2. Calculate the total mass for each element in the compound.
- Pt: 1 × 195.08 = 195.08 amu
- N: 4 × 14.01 = 56.04 amu (4 nitrogen atoms in 4 NH3 groups)
- H: 12 × 1.01 = 12.12 amu (12 hydrogen atoms in 4 NH3 groups)
- Br: 1 × 79.90 = 79.90 amu
- Cl: 3 × 35.45 = 106.35 amu (1 chlorine in the compound + 2 in Cl2)
3. Add the total masses of all the elements to find the molecular mass of the compound.
- Molecular mass = Pt + N + H + Br + Cl
- Molecular mass = 195.08 + 56.04 + 12.12 + 79.90 + 106.35
- Molecular mass = 449.49 amu
To know more about the transition metals https://brainly.com/question/29696009
#SPJ11
Related Questions
PLEASE ANSWER QUICK RIGHT ANSWERS ONLY WILL MARK BRAINLIEST

Answers
Explanation:
To find the freezing point of the solution, we can use the freezing point depression equation:
ΔT = Kᵣ x m
Where ΔT is the change in freezing point, Kᵣ is the freezing point depression constant of benzene, and m is the molality of the solution.
Substituting the values from the problem, we get:
ΔT = 5.12 °C/m x 2.8 m
ΔT = 14.34 °C
Since ΔT = Tᵢ - T, where Tᵢ is the freezing point of the solvent (benzene) and T is the freezing point of the solution, we can rearrange the equation to solve for T:
T = Tᵢ - ΔT
T = 5.50 °C - 14.34 °C
T = -8.84 °C
Therefore, the freezing point of the solution is -8.84 °C.
Answer:The freezing point of the solution is -8.84 °C.
Explanation:
A sample of gas (1.9 mol) is in a flask at 21 °C and 697 mmHg. The flask is opened and more gas is added to the flask. The new pressure is 795 mmHg and the temperature is now 26 °C. There are now __________ mol of gas in the flask.
Answers
Answer:
The new moles of the gas in the flask is 2.13 moles.
Explanation:
Given;
number of moles of gas, n = 1.9 mol
temperature of the gas, T = 21 °C = 21 + 273 = 294 K
pressure of gas, P = 697 mmHg
volume of gas, V = ?
Apply ideal gas law;
PV = nRT
Where;
R is gas constant, = 62.363 mmHg.L / mol. K
V = nRT / P
V = (1.9 x 62.363 x 294) / 697
V = 49.98 L
New pressure of the gas, P = 795 mmHg
New temperature of the gas, T = 26 °C = 273 + 26 = 299 K
New moles of the gas, n = ?
Volume of the gas is constant because volume of the flask is the same when more gas was added.
n = PV / RT
n = (795 x 49.98) / (62.363 x 299)
n = 2.13 moles
Therefore, the new moles of the gas in the flask is 2.13 moles.
The primary mineral that is mined for aluminum. - This is an evaporite mineral mined for its valuable lithium. - Most of this mined mineral is used to melt ice on roadways. A. Calcite B. Bauxite C. Halite D. Spodumene
Answers
The primary mineral that is mined for aluminum is Bauxite. Bauxite is a mineral composed mainly of aluminum hydroxides, typically found in tropical and subtropical regions.
Bauxite is formed through the weathering of rocks in areas with high rainfall. The mineral is typically reddish-brown in color and consists of a mixture of aluminum hydroxides, iron oxides, and other impurities. It is extracted through open-pit mining and then processed to extract aluminum oxide, which is further refined to produce aluminum metal.
Aluminum is a versatile metal with numerous applications in various industries, including construction, transportation, packaging, and electrical equipment. It is lightweight, corrosion-resistant, and has excellent thermal and electrical conductivity. The abundance of bauxite deposits worldwide ensures a steady supply of aluminum for industrial use, making it an essential mineral in the production of this valuable metal.
To know more about Bauxite, visit:
brainly.com/question/30547607
#SPJ11
Select the correct answer. Sami was blowing soap bubbles in his room where the temperature was 23 °C and the pressure was constant. He blew a soup bubble of volume 45 mL. The bubble suddenly escaped from the window where the temperature outside was 12 °C. Explain what will happen to the soap bubble? The volume of the soap bubble will increase to 46.73 mL. The volume of the soap bubble will increase to 86.25 mL. The volume of the soap bubble will decrease to 23.47 mL. The volume of the soap bubble will decrease to 43.33 mL.
Answers
Answer:Question
Select the correct answer. Sami was blowing soap bubbles in his room where the temperature was 23 °C and the pressure was constant. He blew a soup bubble of volume 45 mL. The bubble suddenly escaped from the window where the temperature outside was 12 °C. Explain what will happen to the soap bubble? The volume of the soap bubble will increase to 46.73 mL. The volume of the soap bubble will increase to 86.25 mL. The volume of the soap bubble will decrease to 23.47 mL. The volume of the soap bubble will decrease to 43.33 mL.
Explanation:
Question
Select the correct answer. Sami was blowing soap bubbles in his room where the temperature was 23 °C and the pressure was constant. He blew a soup bubble of volume 45 mL. The bubble suddenly escaped from the window where the temperature outside was 12 °C. Explain what will happen to the soap bubble? The volume of the soap bubble will increase to 46.73 mL. The volume of the soap bubble will increase to 86.25 mL. The volume of the soap bubble will decrease to 23.47 mL. The volume of the soap bubble will decrease to 43.33 mL.
PLEASE HELP
A student labels two 250 milliliter beakers with the letters A and B. She puts 100 milliliters of water in each of them. She adds some salt to beaker A and stirs until the salt dissolves completely. Then she adds another 50 milliliters of water to beaker A. She adds more salt to beaker B, and the salt in beaker B doesn't completely dissolve in the water even after much stirring. The solution in beaker A is The solution In beaker B is

Answers
Answer:
Solution B is saturated and solution A is unsaturated
Explanation:
Saturated solution contains the maximum concentration of a solute dissolved in the solvent (usually water) and if extra solute is added to saturated solution, that solute will not dissolve.
Unsaturated solution means that more of a substance can be dissolved (in this example salt).
When drawing a covalent lewis dot diagram its important to ensure that:
A each atom is surrounded by 6 electrons
B all symbols are in lower case letters
C all the elements have a charge in the top right side
D each atom is surrounded by 8 electrons
Answers
Not sure if it’s correct but Lina positive it is
after the fischer esterification, you will perform liquid-liquid extraction to isolate the ester. how will this be accomplished?
Answers
After the Fischer Esterification, you will perform liquid-liquid extraction to isolate the ester. This can be achieved by mixing the reaction mixture with a non-polar solvent. The two immiscible liquids are then separated using a separating funnel. This process is also known as solvent extraction.
The non-polar solvent extracts the ester from the reaction mixture and is then separated using a separating funnel. Fischer esterification is the chemical process of esterification that involves the reaction of a carboxylic acid with an alcohol to form an ester and a water molecule. This reaction requires a strong acid catalyst, typically concentrated sulfuric acid or hydrochloric acid. Liquid-liquid extraction. Liquid-liquid extraction is a chemical separation process that is based on the distribution of a compound between two immiscible liquids. This process involves the extraction of a desired compound from one liquid phase into another liquid phase by the use of a suitable solvent. The two immiscible liquids are then separated using a separating funnel. This process is also known as solvent extraction.
To know more about Fischer Esterification visit:
https://brainly.com/question/31118260
#SPJ11
how many significant figures are in 0.0231?? a lot of points!!!
Answers
Answer:
3
Explanation:
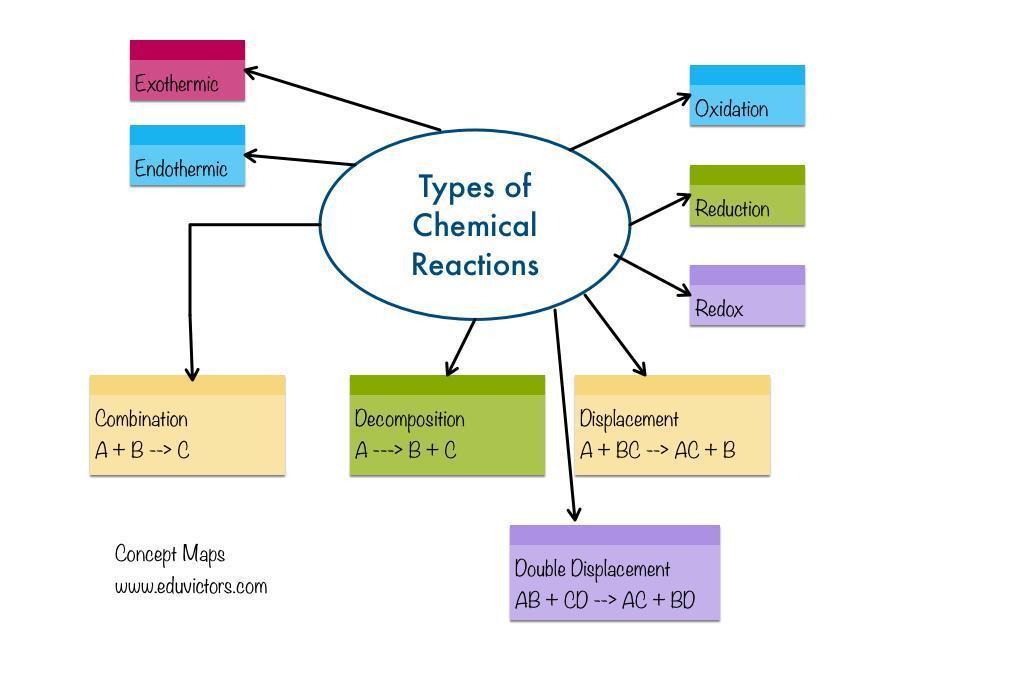
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
how many flourine atoms are in 410 g of UF6
Answers
3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\). Fluorine is an atomic number 9 chemical element with both the symbol F.
What is fluorine?Fluorine is an atomic number 9 chemical element with both the symbol F. This is the smallest halogen as well as occurs as a very poisonous, pale yellow diatomic vapor under normal circumstances.
It is exceptionally reactive being the most electronegative active catalyst, reacting with all other elements save the light inert.
mole = 410 / 352.02 =1.16mole
number of atom= 1.16× 6.022×10²³=6.98×10²³
number of atom of fluorine =6× 6.98×10²³= 3.6 ×10²⁴ atoms
Therefore, 3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\).
To learn more about fluorine, here:
https://brainly.com/question/10700214
#SPJ1
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
how will you know if one sugar is fermented more easily than another?
Answers
When it comes to determining whether one sugar is fermented more easily than another, there are several factors to consider. First, it's important to understand the process of fermentation itself. Fermentation is a metabolic process that converts sugar into alcohol, gases, and organic acids through the action of yeast or bacteria.
The ease of fermentation depends on the chemical structure of the sugar molecule. For example, simple sugars like glucose and fructose are typically easier to ferment than complex sugars like sucrose or lactose. This is because the simpler sugar molecules are easier for the yeast or bacteria to break down and utilize as a food source.
Another factor to consider is the presence of inhibitors or other substances that may interfere with the fermentation process. Some sugars may contain compounds that inhibit the growth or activity of yeast or bacteria, making fermentation more difficult. Conversely, other sugars may contain nutrients or other substances that promote fermentation and make it easier for the microorganisms to thrive.
Ultimately, the best way to determine whether one sugar is fermented more easily than another is to conduct experiments and measure the rate and extent of fermentation under controlled conditions. This may involve monitoring factors like temperature, pH, oxygen levels, and the presence of other microorganisms or substances that could impact the fermentation process. By carefully controlling these variables and comparing the results across different sugar sources, it may be possible to identify which sugars are most easily fermented by a particular type of yeast or bacteria.
Learn more about metabolic process here:
brainly.com/question/19113839
#SPJ11
When 5.97 g of an unknown non-electrolyte is dissolved in 50.0 g of acetone, the boiling point increased by 2.76 degrees C. If the Kbp of the solvent is 1.71 K/m, calculate the molar mass of the unknown solute.
Answers
The molar mass of the unknown solute is 1.4375 g/mol or approximately 1.44 g/mol.
To determine the molar mass of the unknown solute, we can use the formula:
ΔTb = Kbp * m * i
where
ΔTb = boiling point elevation
Kbp = boiling point elevation constant
m = molality of the solution
i = van't Hoff factor.
In this case, we are given the following information:
ΔTb = 2.76°C
Kbp = 1.71 K/m
m = ?
i = 1 (since the unknown solute is a non-electrolyte)
To calculate the molality of the solution using the formula:
m = moles of solute/mass of solvent (in kg)
mass of solute = 5.97 g + 50.0 g = 55.97 g
Converting the mass of the solvent to kg:
mass of solvent = 50.0 g = 0.0500 kg
Now, we can calculate the molality:
m = 55.97 g / 0.0500 kg = 1.12 mol/kg
Finally, we can substitute the values into the boiling point elevation formula and solve for the molar mass of the unknown solute:
ΔTb = (1.71 K/m) * (1.12 mol/kg) * 1
2.76°C = 1.92 K * mol/kg
mol/kg = 2.76°C / 1.92 K = 1.4375
Since mol/kg is numerically equal to the molar mass of the solute, the molar mass of the unknown solute is 1.4375 g/mol or approximately 1.44 g/mol.
know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
what would ebony do in water
Answers
Explanation:
It should be seen that perhaps the intensity of Ebony timber is greater than the concentration. This can drown if this is heavier than water, but that will floating whether it is not viscous than air.
How many moles are in 250 grams of Xenon?
Answers
Answer:
\(1.90\) \(moles\)
Salt is added to water until no more can be dissolved. This is a kind of(blank) solution.
a) Saturated.
b) Unsaturated.
c) Diluted.
d) Insoluble.
Answers
Answer:
The correct answer is:
a) Saturated.
When salt is added to water until no more salt can be dissolved, it forms a saturated solution. A saturated solution is a solution in which the maximum amount of solute has been dissolved at a given temperature and pressure, and no more solute can dissolve. It is in a state of dynamic equilibrium, with solute particles constantly dissolving and precipitating at the same rate.
Please help
urgently!

Answers
1. We can see here that energy is required to change the phase of matter. For example, energy is required to melt ice, vaporize water, and condense steam. The amount of energy required to change the phase of matter is called the latent heat.
What is energy?Energy is a fundamental concept in physics and refers to the ability or capacity of a system to do work or produce a change.
2. The demonstration on the sample of water showed that water can exist in three phases: solid, liquid, and gas. The solid phase is ice, the liquid phase is water, and the gas phase is steam.
The demonstration started with ice at 0°C. As heat was added to the ice, the temperature of the ice increased. However, the ice did not melt until the temperature reached 0°C. This is because the energy from the heat was used to break the bonds between the water molecules in the ice. Once the bonds were broken, the ice melted and became water.
3. Completing the
When all the intermolecular bonds are overcome, the transition between phases is complete. The energy of any substance includes the kinetic energy, potential energy, and thermal energy of its particles.Page 4:
Heating and cooling curves are graphical representations of how temperature changes during the process of heating or cooling a substance. They illustrate the relationship between temperature and the state of matter.
Heating curves represent the temperature changes of a substance as it is heated.Cooling curves, on the other hand, represent the temperature changes of a substance as it is cooled.Both curves show:
Plateaus or flat sections: These occur during phase transitions where the temperature remains constant despite the addition or removal of heat.Changes in slope: The slope of the curve represents the rate of temperature change. Steeper slopes indicate faster changes in temperature, while shallower slopes indicate slower changes.Learn more about energy on https://brainly.com/question/2003548
#SPJ1
To make the future development more eco-friendly and decrease soil erosion,
choose one of the following recommendations:

Answers
Answer:
build better enclosures to decrease overgrazing by livestock.
Explanation:
The fact that water is attracted to itself, a property called , leads to another important property, the liquid form of water is dense than the solid form. As water solidifies into ice, the molecules must move apart in order to fit into a crystal lattice structure, causing water to expand as it freezes. Because of this, and water sinks, which keeps the oceans liquid and prevents them from freezing solid from the bottom up.

Answers
As the first blank is already filled, this first property is called the Cohesive property of water, which is what makes it possible to clump together into drops, due to their intermolecular forces.
The second blank is talking about density, and as we can see in our daily lives, ice is less dense than water, therefore in this blank liquid water will be MORE dense than ice, this is due to the hydrogen bonds and the orientation in which they are causing the molecules to push farther apart.
As we have discussed in the second blank, this 3rd blank is ICE FLOATS
write the balance molecular and net ionic equationf for the reaction between almunimum metal and silver nitrate. identify the oxidation and reduction half-reactions
Answers
The balanced chemical reaction is Al + 3AgNO3 → Al (NO3)3 + 3Ag.
A balanced chemical equation though has the identical number of atoms from every type inside the reaction on both the reactant chemical equation output sides. In a balanced chemical equation, both the mass and the change were equal.
An organic organization may adjust to changes in its surroundings very easily. It is distinguished by low complexity, low centralization, as well as low formalization. A mechanistic organization, on the other hand, is distinguished by great complexity, high centralization, as well as high formalization.
Thus, the balanced chemical reaction will be Al + 3AgNO3 → Al (NO3)3 + 3Ag.
To know more about balanced chemical reaction
https://brainly.com/question/30230799
#SPJ4
Inside a star, the force of gravity is balanced by the:
O A. inward pressure caused by heat.
B. outward pressure caused by spinning.
O c. outward pressure caused by heat.
SUBMIT
Answers
Answer:C
Explanation:outward pressure caused by heat is the force of gravity inside a star.
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Explain, in terms of atomic structure, why Ga has a higher second ionization energy than As?
Answers
Ga has a higher second ionization energy than As due to the removal of an electron from a smaller atomic orbital with greater effective nuclear charge.
Ionization energy is the amount of energy required to remove an electron from an atom or ion in its gaseous state. The second ionization energy is the energy required to remove a second electron from a singly charged ion.
In the case of Ga and As, both elements have the same number of electrons in their outermost shell, but the atomic radius of Ga is smaller than that of As. This means that the valence electrons of Ga are held more tightly by the nucleus due to the greater effective nuclear charge. Therefore, more energy is required to remove a second electron from a Ga ion compared to an As ion, resulting in a higher second ionization energy for Ga.
To know more about the atomic structure, here
brainly.com/question/14467776
#SPJ4
Based on this, which of the reactions below
are double-replacement reactions?
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
2 AgNO3(aq) + Zn(8) + 2Ag(s) + Zn(NO3)2(aq)
Pb(NO3)2(aq) + 2KI(aq) + 2KNO3(aq) + Pb12(s)
BaCl2(aq) + Na2SO4 (aq) + BaSO4(s) + 2NaCl(aq)
Answers
Answer:
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
Explanation:
Chemical reactions are of different types namely; combination reaction, displacement reaction, double displacement reaction etc. Double displacement/replacement reaction is that reaction in which an exchange of ions occur between two reacting ionic compounds to form new products.
In this chemical reaction/equation;
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
2Cl- and SO42- are the ions exchanged in this reaction. Barium (Ba) displaces SO42-, while Sodium (Na) displaces 2Cl-, hence it is called DOUBLE DISPLACEMENT OR REPLACEMENT because the displacement involves two compounds/ions.
In one to two sentences, describe an experiment that would show that intramolecular forces (attractions between atoms within molecules) are stronger than intermolecular forces (attractions between molecules)
Answers
In order to demonstrate that intramolecular forces are stronger than intermolecular forces, a block of ice will be heated in a sealed container until it turns into steam.
Why do intramolecular forces outweigh intermolecular forces?
Because the forces holding together compounds are stronger than the forces holding together molecules, intramolecular forces are stronger than intermolecular forces.
Intermolecular forces exist between molecules, but intramolecular forces exist between atoms within a molecule. This is the primary distinction between intermolecular and intramolecular forces.
Look for the molecule with the most polarity, the most electronegative atoms, or the most hydrogen bonding groups if the molecules have identical molar weights and similar intermolecular forces. That one will have the overall stronger IMFs.
Learn more about intramolecular forces at:
https://brainly.com/question/26096719
#SPJ1
Which element is a metalloid? selenium (Se) germanium (Ge) phosphorus (P) iodine (I)
Answers
Answer:
Ge
Explanation:
Answer:germanium (Ge)
Explanation:
A metalloid is an chemical element that exhibit a mixture of both metals and non metals properties. On the periodic table, they can be found on a zig-zagging line going downwards from the boron to the astatine,dividing nonmetals on the right and the metals on the left. '
They include
boron (B) silicon (Si)germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At).Germanium is hard, has a grayish-white luster appearance and brittle that it can shatter like glass.
...
See related question here:https://brainly.com/question/18738298
Which of the following equations is NOT balanced?A MgCO3→MgO + CO2 B NaCl + KI →NaI + KCl C Na + O2 →Na2O D Ca + MgSO4→CaSO4+ Mg
Answers
Answer: C
Na + O2 →Na2O
Explanation:
A
MgCO3→MgO + CO2 balanced
B
NaCl + KI →NaI + KCl balanced
C
Na + O2 →Na2O not balanced the balanced would be 4Na + O2 →2Na2O
D
Ca + MgSO4→CaSO4+ Mg balanced
Consider the nuclear equation below. 239/94 Pu—-> X+ 4/2 He. What is X?
Answers
Answer:
X = U (Uranium)
Explanation:
Pu-->235/92 U + 4/2 He
The element X which is involved in the given nuclear reaction is ²³⁵₉₂U.
What is radio active decay?Radio active decay is a process in which an unstable nuclei will emit some particles from the nuclei in the form of energy to get stability.
In the given chemical reaction, parent nuclei (²³⁹₉₄Pu) will emit the alpha particle (⁴₂He) which has a atomic number 2 and atomic mass of 4 as a result of which we get a daughter nuclei Uranium which will get the atomic number 92 and atomic mass of 235.
²³⁹₉₄Pu → ⁴₂He + ²³⁵₉₂U
Hence the element X is uranium (²³⁵₉₂U).
To know more about radioactive decay, visit the below link:
https://brainly.com/question/11117468
#SPJ2
Scenario: You are testing two different household solutions with your cabbage indicator, using the color key to the right.
One solution turns blue. A possible hydrogen ion concentration for this solution is: D
1 × 10-2 M
5 × 10-2 M
5 × 10-4 M
1 × 10-8 M
Answers
Answer:
DDDDD
Explanation:
According to the indicator theory, the hydrogen ion concentration for this solution is 1 × 10⁻⁸ M.
What is an indicator?Indicator is defined as a chemical substance which is chemically a weak acid or a weak base which changes it's color depending upon the concentration of hydrogen ions present in the solution.They dissociate slightly in water to produce ions.
These are generally derived from plant pigments and are of slightly acidic or basic in nature.There are three types of indicators:
1) natural indicators
2) synthetic indicators
3) olfactory indicators.
These are mainly used in determination of end point of titrations. Every indicator has it's pH range in which it can perform effectively.These are usually organic compounds.
Learn more about indicators,here:
https://brainly.com/question/29016702
#SPJ7
7 protons 10 electrons 7 neutrons