Two different coils of self inductance L1 and L2 are placed close to each other so that the effective flux in one coil is completely linked with other. If M is the mutual inductance between them, then
Answers
Two different coils of self inductance L1 and L2 are placed close to each other so that the effective flux in one coil is completely linked with other. If M is the mutual inductance between them, then M = √ L1 L2 .
The value of mutual inductance, M for both the coils are :
M = - (e2 / di1 / dt) = - e1 / di2 / dt
where, e1 and e2 are the induced emf , given as :
e1 =- L1 di1 / dt e2 = - L2 di2 / dt
where, L1 and L2 are the self inductance of both the coils,
M² = (e1 e2 ) / ( di1 / dt ) di2 / dt )
M² = L1 L2
M = √ L1 L2
Thus the mutual inductance of the coil is M = √ L1 L2.
To learn more about mutual inductance here
https://brainly.com/question/28585496
#SPJ4
Related Questions
6
What is the density of a substance that has a mass of 2.0 g, and when placed in a graduated cylinder
the volume changed from 70 mL to 75 mL?
A 2.5 g/mL
B 7.0 g/mL
C 10. g/mL
D 0.40 g/mL
Answers
The density of the substance having a mass of 2.0 g is 0.4 g/mL (Option D)
How do I determine the density of the substance?First, we shall obtain the volume of the substance. This can be obtained as follow:
Volume of water = 70 mL Volume of water + substance = 75 mL Volume of substance =?Volume of substance = (Volume of water + substance) - (Volume of water)
Volume of substance = 75 - 70
Volume of substance = 5 mL
Finally, we shall determine the density of the substance. This is illustrated below:
Mass of substance = 2.0 gVolume of substance = 5 mLDensity of substance = ?Density = mass / volume
Density of substance = 2 / 5
Density of substance = 0.4 g/mL
Thus, the density is 0.4 g/mL (Option D)
Learn more about density:
https://brainly.com/question/952755
#SPJ1
What is the molar mass of ammonia gas, NH3? (Round to four significant figures.) 15.00 g 17.01 g 18.00 g
Answers
true or false: u find soil rich in humus in horizon c
Answers
Answer:
True
Explanation:
Calculate the new volume if in a container there is a mass of gas that occupies a volume of 1.3L at a temperature of 280K. Calculate the volume when reaching a temperature of 303K.
Answers
Answer:
1.4 L
Explanation:
From the question given above, the following data were obtained:
Initial volume (V1) = 1.3 L
Initial temperature (T1) = 280 K
Final temperature (T2) = 303 K
Final volume (V2) =?
With the above data, we can obtain the new volume of the gas by using the Charles' law equation as shown below:
V1 /T1 = V2 /T2
1.3/280 = V2 /303
Cross multiply
280 × V2 = 1.3 × 303
280 × V2 = 393.9
Divide both side by 280
V2 = 393.9/280
V2 = 1.4 L
Thus, the new volume of the gas is 1.4 L
how many grams of ice, solid h2o, can be melted with exactly 1.5 kj of heat? the heat of fusion of ice is 6.01 kj/mol, and the molar mass of ice is 18.02 g/mol. express your answer to three significant figures.
Answers
The quantity of ice, solid H₂O, that can be melted with exactly 1.5 kJ of heat, whose heat of fusion is 6.01 kJ/mol, is approximately 4.505 g.
It can be determined through the following steps.
Step 1: Determine the heat of fusion of one mole of ice. The heat of fusion of ice, which is the amount of heat required to melt one mole of ice, is given as 6.01 kJ/mol.
Step 2: Convert the heat required to melt the ice into Joules
1 kJ = 1000 J, so 1.5 kJ = 1500 J
Step 3: Determine the number of moles of ice whose heat of fusion is equivalent to the heat required to melt the ice.
The heat of fusion of 1 mole of ice = 6.01 kJ/mol = 6010 J/mol
The heat required to melt the ice = 1500 J
The number of moles of ice = Heat required to melt the ice/Heat of fusion of one mole of ice
= 1500 J/6010 J/mol ≈ 0.25 mol of ice
Step 4: Convert the number of moles of ice to grams
The molar mass of ice (H2O) = 18.02 g/mol
Mass of ice = Number of moles of ice × Molar mass of ice
= 0.25 mol × 18.02 g/mol≈ 4.505 g
Therefore, the quantity of ice, solid H₂O, that can be melted is approximately 4.505 g.
To know more about the heat of fusion, refer here:
https://brainly.com/question/28876132#
#SPJ11
Which is the limiting reactant when 12.0 mol of CH4 are reacted with 20.0 mol of O2 in the following equation?CH4 + 2 O2 ---> CO2 + 2 H20a. O2b.CO2c. CH4d. H20
Answers
According to the chemical equation, two moles of O2 react with 1 mole of CH4, so the ratio is 2:1. This means if the reaction takes 12.0 moles of CH4, it would react with 24.0 moles of O2. As you can see, we just have 20.0 moles of O2, which means the limiting reactant is the oxygen because CH4 would have a reamining after the reaction.
Therefore, the answer is a. O2.
Calculate the mass of CuO which can react with 39,2 grams of orthophosphate acid.Please Help!!3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
Answers
Answer
47.73 g CuO
Explanation
Given:
Chemical equation: 3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
mass of orthophosphate acid (Cu3(PO4)2) = 39.2 g
Required: The mass of CuO
Solution:
\(\begin{gathered} 39.2g\text{ H}_3PO_4\text{ x }\frac{1\text{ mol H}_3PO_4}{97,994g\text{ H}_3PO_4}\text{ x }\frac{3\text{ moles CuO}}{2\text{ moles H}_3PO_4}\text{ x }\frac{79,545g\text{ CuO}}{1mole\text{ CuO}} \\ \\ =\text{ 47.73 g CuO} \end{gathered}\)Second method:
Step 1: Find the moles of H3PO4
n = m/M where m is the mass and M is the molar mass of H3PO4
n = 39.2g/97.994g.mol^-1
n = 0.400 mol
Step 2: Use the stoichiometry to find the moles of CuO
The molar ratio between CuO and H3PO4 is 3:2
Therefore the moles of CuO = 0.400 mol x (3/2) = 0.600 mol
Step 3: Find the mass of CuO, now that we have moles
m = n x M m is the mass, n is the moles and M is the molar mass
m = 0.600 mol x 79,545 g/mol
m = 47.73 g
a(n) _____ is a chemical combination of two or more atoms in definite (fixed) proportions.
Answers
A(n) molecule is a chemical combination of two or more atoms in definite (fixed) proportions.
Molecules are formed when atoms bond together in specific ratios.
These ratios are determined by the atoms' valence electrons and their ability to form stable bonds.
Molecules can consist of atoms of the same element, like O2 (oxygen gas), or atoms of different elements, like H2O (water).
A molecule is a chemical combination of two or more atoms in definite proportions, resulting from the stable bonding of atoms based on their valence electrons.
Learn more about molecule click here:
https://brainly.com/question/475709
#SPJ11
What will be the pressure if the temperature is lowered to 21.663 Celsius

Answers
1.73 atm will be the pressure if the temperature is lowered to 21.663 Celsius. The correct option is C.
Thus, the coupled gas law, which states that the product of pressure and volume is exactly proportional to the absolute temperature, may be used to calculate the pressure of the gas at 21.663 degrees Celsius. If the volume stays constant, the pressure of the gas will likewise fall correspondingly as the temperature drops.
We may use the proportionality relationship to compute the final pressure using the beginning circumstances of 2.1 atm pressure, 3.78 L volume, 82°C temperature, and 21.663°C temperature. Due to the drop in temperature, the final pressure will be 1.73 atm lower than the beginning pressure.
Thus, the ideal selection is option C.
Learn more about pressure here:
https://brainly.com/question/18431008
#SPJ1
after an organic reaction involving an aqueous solution, the organic solution might be washed with a saturated sodium chloride aqueous solution, known as brine. what is the purpose of the brine wash? select one: to increase the density of the organic solution to reduce the amount of water in the organic solution to reduce the volume of the organic solution to remove organic solvent, isolating a solid product
Answers
The purpose of the brine wash is to reduce the amount of water in the organic solution.
Brine or high concentration of sodium chloride in water often finds application in industrial processes to remove impurities and other foreign and unwanted substances form the yields. It can easily remove the water due to its high affinity with water.
The same is achieved through high osmotic gradient formed by high concentration of solute particles in the brine, which causes flow of water thus drying up the organic solution.
Learn more about brine -
https://brainly.com/question/19557011
#SPJ4
given a balanced chemical equation between h2so4(aq) and koh(aq) h2so4(aq) 2 koh(aq) → k2so4(aq) 2 h2o(l) what volume (in ml) of 0.78 m h2so4(aq) solution is necessary to completely react with 106 ml of 0.47 m koh(aq)? note: (1) the unit of volume of h2so4(aq) is in ml (2) insert only the numerical value (integer) of your answer (do not include the units or chemical in your answer).
Answers
The volume of 0.78 M H2SO4(aq) solution necessary to completely react with 106 ml of 0.47 M KOH(aq) is approximately 128 ml.
To find the volume of 0.78 M H2SO4(aq) solution necessary to react with 106 ml of 0.47 M KOH(aq), we can use the concept of stoichiometry.
From the balanced chemical equation,
First, let's find the number of moles of KOH in 106 ml of 0.47 M KOH(aq):
0.47 moles/L x 0.106 L = 0.04982 moles of KOH
Since the mole ratio is 1:2, we need double the amount of H2SO4.
2 x 0.04982 moles = 0.09964 moles of H2SO4
Next, let's calculate the volume of 0.78 M H2SO4(aq) solution containing 0.09964 moles of H2SO4:
Volume (in L) = Moles / Molarity
= 0.09964 moles / 0.78 moles/L
= 0.12774 L
To convert this to milliliters (ml), we multiply by 1000:
0.12774 L x 1000 = 127.74 ml
To know more about volume visit:-
https://brainly.com/question/33501668
#SPJ11
(science)
HURRY 5 MINUTES
What kind of drug is nicotine? a. anabolic b. depressant c. stimulant d. inhalant
Answers
Answer:
Nicotine is classified as a stimulant drug.
Why does the potato lose mass as molarity increases
Answers
When the molarity of the surrounding solution is higher than that inside the potato cells, water moves out of the cells, causing the potato to lose mass.
What is molarity?Potatoes are made up of cells, and these cells contain water and dissolved substances such as starch, sugars, and minerals. When a potato is placed in a solution with a higher molarity than the potato's cells, water moves out of the cells via osmosis. Osmosis is the movement of water molecules from an area of high water concentration to an area of low water concentration across a semipermeable membrane, such as a cell membrane.
The higher molarity solution has a lower water concentration than the potato cells, so water moves out of the cells and into the surrounding solution. This causes the potato to lose mass because the water leaving the cells is no longer contributing to the overall weight of the potato.
When the molarity of the surrounding solution is higher than that inside the potato cells, water moves out of the cells, causing the potato to lose mass.
Learn more about Molarity
brainly.com/question/31751020
#SPJ11
If two gases react, pumping more gas into the reaction container will _____ the rate of the reaction.
A. increase
B. decrease
Answers
Answer:
increase the rate of reaction
Light energy constitutes minute packets discrete from one another called?
Answers
Light energy constitutes minute packets discrete from one another called as photons.
The Light energy is the electromagnetic radiation. It will exists in the discrete packets of the energy called as the quanta, or the photons. The Photon cannot be subdivided, and the consequently light will always consists of the integer number of the photon. The photon will differ from the each other in the amount of the energy they contain.
The expression for the energy of the photon is as :
E = hv
Where
v is the frequency of light incident.
h is the Planck's constant
Thus , photons is light energy that constitutes minute packets discrete from one another.
To learn more about photons here
https://brainly.com/question/14758126
#SPJ4
concentrated hcl is a 12.0 m solution in water and has a density of 1.22 g/ml. a. what is the molality of hcl in the solution?
Answers
The molality of HCl in the solution is\(0.00122 mol/kg\)
Molality is defined as the number of moles of a solute in one kilogram of a solvent. In this case, the solvent is water.
To calculate the molality, we first need to calculate the molarity of the solution. Molarity is defined as the number of moles of a solute in one liter of solution.
To calculate the molarity, we need to know the mass of the solute (HCl) and the volume of the solution. The mass of the solute can be calculated using the density of the solution (1.22 g/ml):
Mass of HCl =\(Density * Volume\)
Volume = \(\frac{Mass}{Density }\)
Volume =\(\frac{(12.0 mol/L) }{ (1.22 g/ml) }= 9.839 L\)
Molarity = \(\frac{ (12.0 mol/L) }{(9.839 L) }= 1.22 mol/L\)
Now, we can calculate the molality:
Molality = \(\frac{ (1.22 mol/L) }{(1000 g/kg) }= 0.00122 mol/kg\)
Therefore, the molality of HCl in the solution is \(0.00122 mol/kg\).
learn more about Molality Refer:brainly.com/question/26921570
#SPJ4
Correctly explain why shower curtains move using the principles of Boyle's law
Answers
Answer:
The pressure on the shower side of the curtain will be lower than the pressure on the outside at the same height from the floor, causing the curtain to move toward the lower pressure. The problem with this explanation is that the curtain will suck inward toward a cold shower, too.
Which of the following statements is true

Answers
Answer:
Solods have a fixed mass, volume,and shape
A student builds an electrical circuit. In the circuit, a
battery is connected to a siren.
Which series of energy transformations takes place in the circuit when the
siren turns on?
A. Chemical energy - Sound energy - Electrical energy
O B. Electrical energy - Chemical energy - Sound energy
C. Chemical energy - Electrical energy - Sound energy
O D. Sound energy - Electrical energy - Chemical energy
Answers
Answer:
C. Chemical energy - Electrical energy - Sound energy
Explanation:
According to the law of conservation of energy, energy can be transformed i.e changed from one form to another. According to this question, a student builds an electrical circuit, which connects a battery to a siren. Based on this description, the electrical circuit uses ELECTRICAL ENERGY supplied by the CHEMICAL ENERGY in the battery cell.
The electrical energy in the circuit connects the CHEMICAL ENERGY in the battery to the siren, which makes the sound using SOUND ENERGY. In other words, the CHEMICAL ENERGY of the battery powers the ELECTRICAL ENERGY of the circuit to make SOUND ENERGY in the siren. Hence, the order of energy transformation is: Chemical energy - Electrical energy - Sound energy
Answer:
C. Chemical energy - Electrical energy - Sound energy
Explanation: I took the test:)
Question 8 of 10
Which of the following is made of matter?
A. Light
B. Energy
C. The sun
D. Heat
Answers
Answer: i Choose the sun
because it has mass
Explanation: matter is anything that has mass and occupies space / Volume
and the sun has mass though it is not a solid one, and is made up of matter
HOPE THIS HELPSS!!!!
what safety equipment do you need if you are working with a strong base
Answers
Answer:
Closed-toe shoes, long pants, a lab coat, safety glasses with side shields or splash goggles, and gloves.
Explanation:
What is the density of gold if 97.3 cm^3 has a mass of 1880 grams?
Answers
Answer:
Explanation:
the equation is d=m/v
convert cm to m
97.3*100=9730m^3
d=1880g/9730cm^3
d=0.19g/m^3
If the collected information showed that the temperature was 10°C and the wind speed was 75 mph, what type of weather would it
probably be outside?
OA. cold and wet
OB. cold and windy
OC. warm and windy
OD. warm and sunny
Answers
Answer:
cold and windy
Explanation:
if its 10 degrees outside then it must be cold. then 75 mph that is very windy
When Oxygen Accepts Electrons, Water Is Produced As A Byproduct.True/False
Answers
False. When oxygen accepts electrons, it does not produce water as a byproduct. Water is formed when oxygen atoms combine with hydrogen atoms through a chemical reaction known as hydrogenation.
The statement that water is produced as a byproduct when oxygen accepts electrons is incorrect. Water is composed of two hydrogen atoms and one oxygen atom, and it is formed through the chemical reaction known as hydrogenation.
In this reaction, hydrogen atoms are added to oxygen, resulting in the formation of water molecules. The process of oxygen accepting electrons is referred to as reduction, and it can occur in various chemical reactions, but the byproduct of this process is not water. It is important to differentiate between the role of oxygen in accepting electrons and the specific chemical reaction that produces water.
To learn more about atoms click here:
brainly.com/question/1566330
#SPJ11
the value of the ionization constant, ka, for hypochlorous acis, hocl is 3.1x10^-8. calculate the hydronium ion concetration
Answers
The hydronium ion concentration for hypochlorous acid (HOCl) is 1.66 x 10^-5 M.
To calculate the hydronium ion concentration for hypochlorous acid (HOCl), we can use the ionization constant (Ka) expression:
Ka = [H3O+][OCl-] / [HOCl]
Since the ionization of HOCl is a 1:1 ratio, we can assume that the concentration of H3O+ (hydronium ions) is equal to the concentration of OCl- (hypochlorite ions). Let's represent the hydronium ion concentration as x:
3.1 x 10^-8 = (x)(x) / (1-x)
To simplify the calculation, we can assume that x is much smaller than 1, so 1-x ≈ 1:
3.1 x 10^-8 = x^2
Now, take the square root of both sides to find x:
x = √(3.1 x 10^-8) ≈ 1.66 x 10^-5 M
Thus, the hydronium ion concentration is 1.66 x 10^-5 M.
Learn more about ionization constant here:
https://brainly.com/question/30639622
#SPJ11
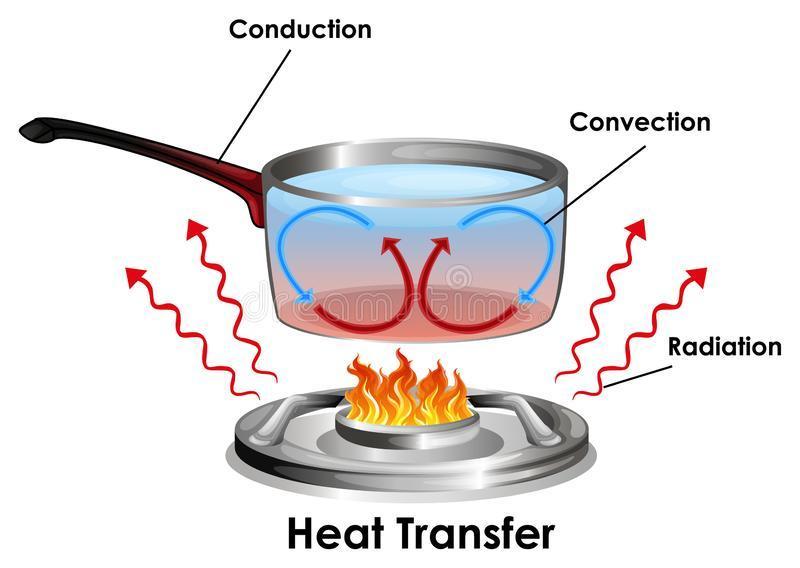
The diagram shows an example of convection.
A picture of a clear pot of boiling water on a stove. There is an arrow pointing at the flame.
Which label belongs on the arrow?
Answers
From the diagram showing an example of convection, the label that belongs on the arrow is cooler water sinks while hotter water rises.
What are convection currents?Convection currents are currents that are set up by particles of a substance that transfer heat by the process of convection.
Convection is a form of heat transfer in which the particle of the substance transferring heat move from one point to another transferring heat as they move.
Convection is one of the three processes of heat transfer. The two other processes of heat transfer are conduction and radiation.
In the diagram shown, the heat transfer process show is convection.
The heat supplied by the flame is transferred from the bottom of the pot to the top by the movement of the water molecules. Heated water molecules move up while cold water molecules move down to replace them, thus, setting up a convection current.
Learn more about convection at: https://brainly.com/question/25957304
#SPJ1
You are performing an experiment and produce an unidentified compound. You know it is composed of carbon, hydrogen, and oxygen but you do not know how many of each are present. You determine its relative formula mass is 59 g/mol. What is its molecular formula?
A. C2H3O2
B. C4H3O4
C. CH12O2
D. C3H4O
Answers
Answer:
C₂H₃O₂
Explanation:
Given the relative formula mass = 59g/mol
Unknown:
Molecular formula of the compound
Solution:
To identify this compound, let us find the molecular mass of the choices given.
The one that tallies with the number 59 is the solution:
For;
C₂H₃O₂;
Atomic mass of C = 12
Atomic mass of H = 1
Atomic mass of O = 16
Insert the parameters and solve;
Relative molecular formula = 2(12) + 3(1) + 2(16) = 59g/mol
So, the first choice is the compound.
What is the formula for potassium nitrate? (3 points) a KN b K3N c KNO2 d KNO3
Answers
Answer:
KNO3 (Potassium Nitrate)
Explanation:
I just know.
What particles are used to calculate the atomic mass *?
Answers
The number of protons and the number of neutrons determine an element’s atomic mass: mass number = protons + neutrons.
Atoms of each element contain a characteristic number of protons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); the number of protons in an atom is called the atomic number. In contrast, the number of neutrons for a given element can vary. Forms of the same atom that differ only in their number of neutrons are called isotopes. Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom’s mass number is its atomic mass. The atomic mass of a single atom is simply its total mass and is typically expressed in atomic mass units or amu. By definition, an atom of carbon with six neutrons, carbon-12, has an atomic mass of 12 amu. Other atoms don’t generally have round-number atomic masses for reasons that are a little beyond the scope of this article. In general, though, an atom's atomic mass will be very close to its mass number, but will have some deviation in the decimal places. Since an element’s isotopes have different atomic masses, scientists may also determine the relative atomic mass—sometimes called the atomic weight—for an element. The relative atomic mass is an average of the atomic masses of all the different isotopes in a sample, with each isotope's contribution to the average determined by how big a fraction of the sample it makes up. The relative atomic masses given in periodic table entries—like the one for hydrogen, below—are calculated for all the naturally occurring isotopes of each element, weighted by the abundance of those isotopes on earth. Extraterrestrial objects, like asteroids or meteors, might have very different isotope abundances.
Learn more about atomic mass here:
https://brainly.com/question/8101390
#SPJ4
the neutralization reaction gets its name from.the fact that the products of the reaction are
Answers
Answer:
Neutral
Explanation:
Neutralization reaction gets its name from the fact that the products of the reaction are _____.
neutral