Use the bond energies to answer the question.
H–H = 432
H–O = 467
O=O = 495
Two H2 molecules combine with one O2 molecule, forming two water molecules (H2O) bonded as such: H–O–H. Which option shows the difference in total bond energy between the reactants and the products?
1,359
–7
–509
1,868
Answers
The change in bond energy here, is the difference between the sum of bond energies of reactants and that of products. Thus, the correct option is -509.
What is bond energy ?Bond energy is the energy required to break the bond between two atoms in a molecule or compound. The change in bond energy in a reaction is the difference between the sum of bond energies of reactants and that of products.
Given,
H - H = 432
for 2 H - H = 864
O - O = 495.
The reaction is combination of two hydrogen molecules with one oxygen molecule to form two water molecules.
One water molecule contains two O-H bonds. Hence two water molecules contains 4 O-H bonds.
O-H= 467
Then 4 O - H = 1868.
Then, the total bond energy between reactants and products is:
864 + 495 - 1868 = -509.
Therefore, option c is correct.
Find more on bond energy:
https://brainly.com/question/26141360
#SPJ1
Related Questions
Areas of the earth with lots of direct sunlight have warm air.explain why warm air rises and leaves pockets of low pressure behind ?
Answers
Answer: Equator
Explanation:
The equator and the regions near the equator receive maximum solar radiation every year. These areas exhibit warm air. The warm air rises and the cools down then further at low pressure zone it might not be able to hold the water vapors. So the water vapors present in the air will cool down and condense in the form of clouds and the rain fall down.
what are the impact of soil Science to the development of Ghana's agriculture?
Answers
In an ecosystem, the impact of soil Science to the development of Ghana's agriculture is that it provides suitable conditions for root germination and growth.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ9
The equilibrium constant for a particular reaction has been measured at different temperatures. The results are plotted below: n A I/T Determine the correct thermodynamic properties for this reaction:(Warning!: There is a maximum of 2 attempts for this question) O endothermic with Aso o O exothermic with ASo < 0 O exothermic with aso > 0 O endothermic with ASo o O more information is needed Submit AnswerIncorrect. Tries 1/2 Previous Tries
Answers
Therefore, option an is the best choice and the equilibrium constant is 0.32. The best choice is that.
The equilibrium constant's value falls as temperature rises. An rise in temperature increases the value of the equilibrium constant when the forward reaction is endothermic. As the temperature fluctuates, so does the equilibrium position. For elements in their standard condition, G0f G f 0 is taken into consideration as zero. As a result, the reaction's standard change in Gibb's free energy at 25 degrees Celsius is 98.746 kJ. Since there is no longer any free energy to fuel the process at equilibrium, G=0.
Learn more about reaction here-
https://brainly.com/question/28984750
#SPJ4
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Predict the crystal ionic radius for antimony, Sb, observing the trend in values for the crystal ionic radii of the other elements belonging to the same group. p .340, As .580, Sb
Answers
Explanation:
We know that Ionic radius increases on going downwards in any group. In group 15, the last elements Sb and Bi have ionic radii lesser than the rest of its elements i.e N,P and As.
This is due to the fact that Sb and Bi have completely filled d and f sub-shells which have least shielding effect.
Thus, they do not guard the valence electrons to a greater extent due to which the valence electrons feel almost whole nuclear charge.
This, results into attraction of valence electrons towards nucleus and hence size decreases.
You analyze a cell in your microscope likely effect of this cellular defect?
that is missing most of its ribosomes. What is the Likely effect of this Cellular defect?
Answers
Answer:
This question has no options but it can be answered based on the understanding of the function of ribosomes.
The effect is the deficiency of proteins in the cell.
Explanation:
Ribosomes are one of the most important cellular organelles found in living organisms. Infact, they are so important that they are found in both the prokaryotic and eukaryotic cell. Ribosome functions in PROTEIN SYNTHESIS as they are the site where the mRNA encoding protein building blocks (amino acids) undergo their translational process.
According to this question, an analyzed cell is found to be missing most of its ribosomes. This means that the function performed by the missing organelle, which is SYNTHESIS OF PROTEINS, will be lacking. Hence, that particular cell will be PROTEIN-DEFICIENT.
What is Specific Heat Capacity?
DR0uQu
Answers
Answer:
Specific heat capacity is a measure of how much energy is needed to raise the temperature of a material and is defined as follows:
Is a dish sponge a heterogenous mixture or homogenous mixture
Answers
Answer:
Heterogeneous mixture.A dish sponge is considered a heterogeneous mixture. A heterogeneous mixture is one that consists of visibly different components or substances that are not uniformly distributed throughout the mixture. In the case of a dish sponge, it is typically made up of a porous material, such as a sponge or foam, which is combined with other components like detergents or cleaning agents. These different components can be observed as distinct regions or phases within the sponge, making it a heterogeneous mixture.
i need help answering number 1 and number 3 50 points!!

Answers
The removal of hydrogen or any other electropositive element, or the addition of oxygen, is said to be the process of oxidation in classical or earlier concepts. An atom or ion gains one or more electrons during the process of reduction.
1. The oxidation half-reaction of copper is:
Cu → Cu²⁺ + 2e⁻
The reduction half is:
Cu²⁺ + 2e⁻ → Cu
3. An anode in electrochemistry is, in its simplest form, the site of an oxidation reaction. Due to the anode's electrical potential, negative ions or anions usually react there and release electrons. After that, these electrons ascend and enter the drive circuit.
In chemistry, the cathode is referred to as the electrode where reduction takes place. In an electrochemical cell, this is typical. Here, the cathode is negative because the cell's electrical energy supply causes chemical molecules to break down.
To know more about anode, visit;
https://brainly.com/question/17109743
#SPJ1
Persistent organic pollutants (POPs) should constantly be monitored because they can bioaccumulate in the tissues of organisms.
True False
Answers
Answer:
true
Explanation:
POPs bio-magnify throughout the food chain and bio-accumulate in organisms.
True. Persistent organic pollutants (POPs) are a group of chemicals that are characterized by their resistance to environmental degradation.
These pollutants can persist in the environment for long periods of time and can accumulate in the tissues of living organisms through a process known as bioaccumulation. Bioaccumulation occurs when organisms absorb these pollutants from their surroundings at a rate that exceeds their ability to eliminate them.
POPs are of particular concern because they can have harmful effects on both the environment and human health. As they accumulate in the tissues of organisms, they can disrupt biological processes and lead to a variety of adverse health effects, including developmental, reproductive, and neurological disorders.
Monitoring of POPs is crucial because it helps to assess their levels in the environment and track any potential changes or trends. By monitoring POPs, scientists and policymakers can gain insights into the extent of contamination, identify potential sources of exposure, and make informed decisions about management and regulatory actions. Monitoring efforts may include measuring POP levels in air, water, soil, and biological samples, as well as assessing their impact on different ecosystems and species.
In conclusion, the statement is true. Persistent organic pollutants (POPs) should be constantly monitored due to their potential to bioaccumulate in the tissues of organisms and their associated adverse effects on both the environment and human health.
To learn more about Persistent organic pollutants, here
https://brainly.com/question/25776069
#SPJ3
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
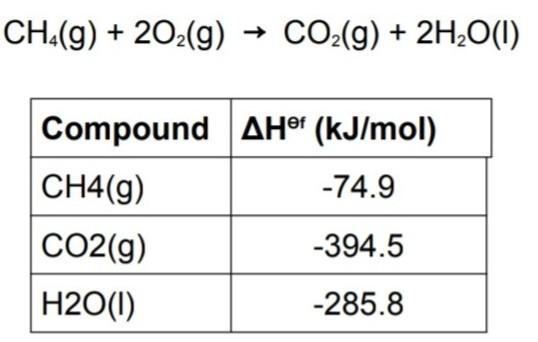
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
In a robot, what is an actuator
Answers
Answer:
devices that move a robot's joints
Explanation:
how many molecules of potassium chloride will react if 21.89 grams KCl are added to the solution
Answers
There are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
What is meant by potassium chloride ?Potassium chloride (KCl) is a compound made up of potassium and chloride ions. It is a colorless, odorless salt that is commonly used in a variety of applications.
Molar mass of KCl is 74.55 g/mol; number of moles = Mass/ Molar mass
So, the number of moles = 21.89 g ÷ 74.55 g/mol = 0.2936 mol
and the number of molecules = Number of moles * Avogadro's number
Number of molecules = 0.2936 mol x 6.02 x 10²³ molecules/mol
Number of molecules = 1.765 x 10²³ molecules
Therefore, there are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1
how was the periodic table organized the second time?
Answers
Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. nickel(II) cyanide silver hydroxide
Answers
Answer:
\(Ksp=[Ni^{2+}][CN^-]^2\\\\Ksp=[Ag^+][OH^-]\)
Explanation:
Hello there!.
In this case, for this equilibrium problem, it turns out firstly necessary to write the chemical equations for the solubilization of both nickel (II) cyanide and silver hydroxide as shown below:
\(Ni(CN)_2(s)\rightarrow Ni^{2+}(aq)+2CN^-\\\\AgOH(s)\rightarrow Ag^+(aq)+OH^-\)
Thus, by means of the law of mass action we set up these equilibrium expressions as shown below:
\(Ksp=[Ni^{2+}][CN^-]^2\\\\Ksp=[Ag^+][OH^-]\)
Best regards!
if naphthalene is mixed with alcohol does it become a homogeneous or heterogeneous mixture?
Answers
When naphthalene is mixed with alcohol does it become a homogeneous mixture.
What is a homogeneous mixture?A homogeneous mixture is the type of mixture that is obtained between two substances when mixed such that a uniform distribution is observed.
While a heterogeneous mixture is the type of mixture that is obtained between two substances such that non uniform distribution is observed.
An example of a heterogeneous mixture is the mixture of sand and water or oil and water. This leads to the formation of two distinct layers.
Naphthalene can be dissolved in alcohol in such a way that a uniform mixture is obtained. Therefore when mixed a homogeneous mixture is obtained.
Learn more about mixture here:
https://brainly.com/question/24647756
#SPJ1
Calculate the molarity of a solution that contains 183.51 grams of lead (II) bromide in 500.0 mL of the solution
Answers
Answer: 1M
Explanation:
Molarity = mols/L
moles of lead bromide: 183.51/ 367.0 = 0.5 mol
500 ml/ 1000 mL = .5L
.5 mol / .5 L = 1 mol/L = 1M
out of all of the planetary health measures shown in the images provided which one shows if humans have already exceeded?A. global average surface temperature B. ocean ph C. atmospheric carbon dioxide D. sea level

Answers
According to the picture, Global average surface temperature, is in extreme levels and shows that human actions contributed to this increase in global temperature.
Consider the reaction of zinc metal with hydrochloric acid:
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g).
If 29.39 × 1024 atoms of zinc completely reacted with hydrochloric acid, how many moles of hydrochloric acid must have reacted?
Do NOT include units in your entry. Report your answer with 3 SFs.
______________________ moles of HCl
Answers
Answer:
6.054×10²⁵
Explanation:
1)find number of moles of zinc
2)multiply the mole of zinc with 2
3)use the formula mol = number of particle/ avogadro constant
Total, 97.64 moles of hydrochloric acid (HCl) must have reacted.
To determine the number of moles of hydrochloric acid (HCl) that reacted, we first need to find the molar ratio between zinc (Zn) and hydrochloric acid (HCl) from the balanced chemical equation:
Zn(s) + 2 HCl(aq) → ZnCl₂(aq) + H₂(g)
From the equation, we see that 1 mole of Zn will reacts with 2 moles of HCl.
Given that 29.39 × 10²⁴ atoms of zinc reacted, we need to convert this quantity to moles. We can do this by using Avogadro's number:
1 mole of any substance = 6.022 × 10²³ atoms
Number of moles of zinc reacted = (29.39 × 10²⁴ atoms) / (6.022 × 10²³ atoms/mol)
Number of moles of zinc reacted ≈ 48.82 moles (rounded to 3 significant figures)
Now, using the molar ratio from the balanced equation, we can determine the number of moles of hydrochloric acid (HCl) that reacted:
Number of moles of HCl reacted = 2 × Number of moles of zinc reacted
Number of moles of HCl reacted ≈ 2 × 48.82 moles
≈ 97.64 moles
Therefore, approximately 97.64 moles of hydrochloric acid (HCl) must have reacted.
To know more about hydrochloric acid here
https://brainly.com/question/14519330
#SPJ2
Which of the following is a radioactive element?Sodium, Fluorine,Oxygen
francium
Answers
Answer:
Fluorine
Explanation:
These particles stick in the atoms and make them radioactive.
The helium tank has a pressure of 650 torr at 25 degree celsius what will be the pressure if the temperature is tripled?
pa help po
Answers
The helium tank has a pressure of 650 torr at 25 degree Celsius and when the temperature is tripled, the pressure will be approximately 1945.71 torr
To find the new pressure when the temperature is tripled, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and the number of particles remain constant. The ideal gas law is given by the equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 degrees Celsius to Kelvin. Adding 273.15 to the Celsius temperature gives us 298.15 K.
Let's assume that the volume, number of moles, and the gas constant remain constant.
If the temperature is tripled, the new temperature would be 3 times the initial temperature, which is 3 * 298.15 K = 894.45 K.
Now, we can set up a proportion to find the new pressure:
P1 / T1 = P2 / T2
Solving for P2 (the new pressure), we get:
P2 = (P1 * T2) / T1
Plugging in the values, we have:
P2 = (650 torr * 894.45 K) / 298.15 K
Calculating this expression, we find:
P2 ≈ 1945.71 torr
Therefore, when the temperature is tripled, the pressure will be approximately 1945.71 torr.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
Part D What evidence can be used to support the fact that oxidation, reduction, or both took place in test tube 5?
Answers
Copper atoms (Cu) were created by reducing copper ions (Cu2+). An illustration of a reduction reaction is this. (Mg2+).To create Mg2+ ions, Mg atoms lost their electrons. An example of an oxidation reaction is this.
Oxidation and reduction occurred in test tube 5. Here is the equation for the reaction that took place:MgSO4 (aq) + Cu (s) CuSO4 (aq) + MgCuSO4's blue hue was neutralised to a solid copper hue. Copper atoms (Cu) were created by reducing copper ions (Cu2+). An illustration of a reduction reaction is this. Cu atoms are created when Cu2+ gains electrons. Due to the presence of magnesium ions (Mg2+), the solution was greenish-yellow.To create Mg2+ ions, Mg atoms lost their electrons. An example of an oxidation reaction is this. Two electrons were lost by the magnesium atom, leaving two electrons behind.
As a result, test tube 5 experienced both oxidation and reduction.It is possible to prove that oxidation, reduction, or both took place through the reaction's electron transfer and colour changes. A reduction reaction was seen when copper ions transformed into copper atoms. An oxidation reaction was evident when magnesium atoms transformed into magnesium ions.
For more question on reaction
https://brainly.com/question/11231920
#SPJ8
Please help with this chemistry question

Answers
the cell potential for the given galvanic cell is +3.16 V at 25°C. This means that the reaction is strongly favored to occur spontaneously in the direction written, from left to right.
To calculate the cell potential for the given galvanic cell, we need to use the standard reduction potentials of the two half-reactions involved in the cell reaction. From the table of standard reduction potentials, we can find the following half-reactions:
Al³+ (aq) + 3e⁻ → Al(s) E°red = -1.66 V
Au³+ (aq) + 3e⁻ → Au(s) E°red = +1.50 V
To obtain the overall cell potential, we can use the equation:
E°cell = E°reduction (cathode) - E°reduction (anode)
where the cathode is the half-cell where reduction occurs and the anode is the half-cell where oxidation occurs. In this case, we can see that Au³+ is reduced to Au(s) (cathode) and Al(s) is oxidized to Al³+ (anode).
Therefore, we can calculate the cell potential as follows:
E°cell = E°red (Au³+ → Au) - E°red (Al → Al³+)
= (+1.50 V) - (-1.66 V)
= +3.16 V
Thus, the cell potential for the given galvanic cell is +3.16 V at 25°C. This means that the reaction is strongly favored to occur spontaneously in the direction written, from left to right.
To know more about reduction potentials, visit:
https://brainly.com/question/23881200
#SPJ1
This image represents what?
A) Atom of nitrogen
B) mixture of nitrogen atoms
C) compound containing nitrogen
D) molecule of diatomic nitrogen

Answers
Answer:
IT’S D)molecule of diatomic nitrogen
Explanation:
Thx to the person above though..........
Answer:
D
Explanation:
Name the following lonic Compounds using the lonic naming rules. Remember, place the metal's name
first, followed by the non-metal element, replacing the ending with "-ide"
1.Caci,
2.LIBr
I
3. Bes
4. LIF
5. K Se
6. Sr,P2
7. Baci
8. Feo
9. Fe,
10. CUN
11. Cun,
Please help meeee
Answers
2.Lithium Bromide
3.Beryllium Sulfide
4.Lithium Fluoride
5. Potassium hydroselenide
6. Strontium phosphide
7.Barium Chloride
8.Iron Oxide
9.Iron
10.?
11.Copper Nitride
Need help with this
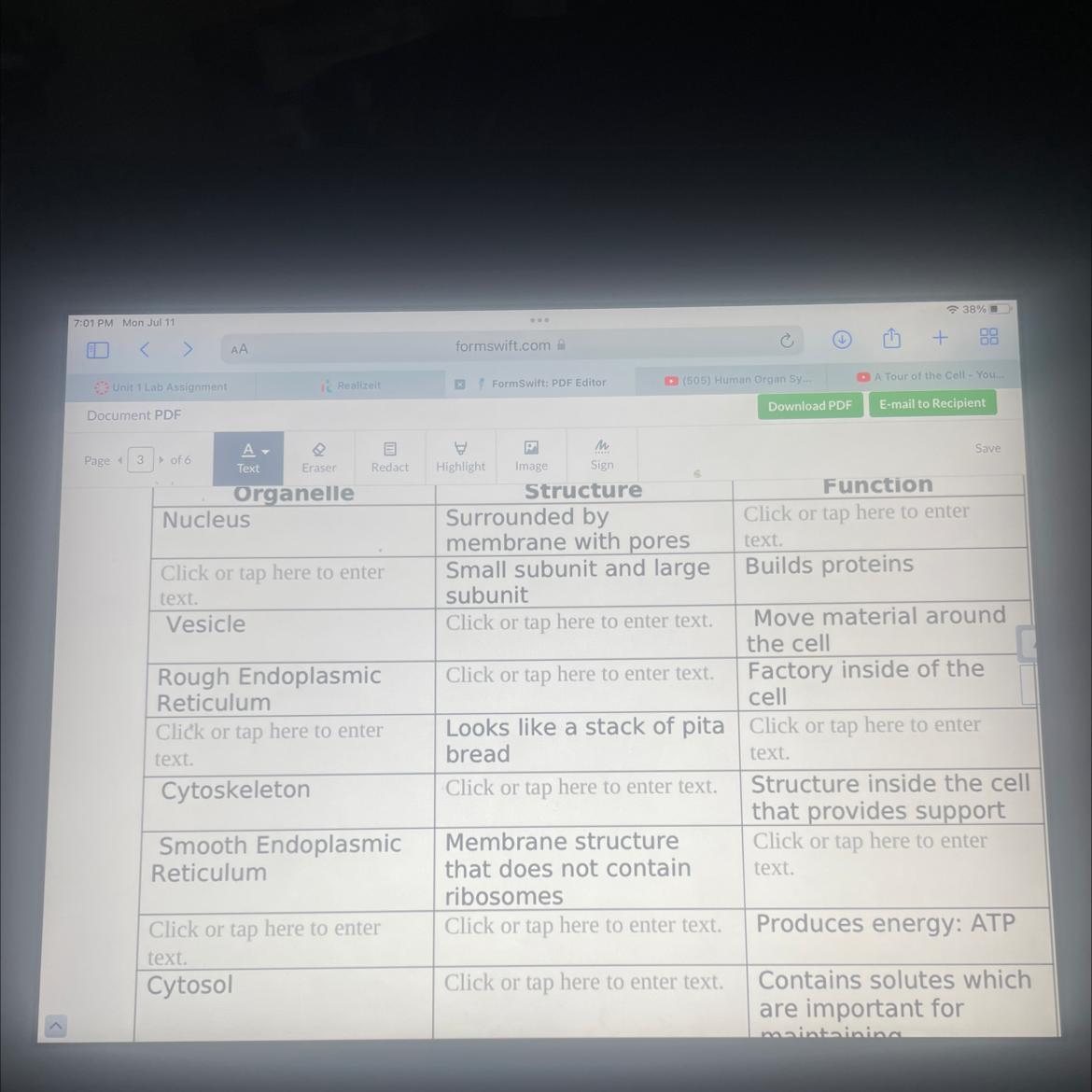
Answers
Answer:
- Nucleus Function - Controls and regulates the activity of the cell.
- Ribosome builds proteins.
- A vesicle structure is kinda like a sac filled with a fluid of sorts.
- Rough ER has ribosomes attached to the cytoplasmic side of the membrane.
- Golgi apparatus kinda looks like a "stack of peta bread"
- Golgi apparatus functions as a factory in which proteins received from the ER are further processed and sorted for transport to their eventual destinations
The cytoskeleton of a cell is made up of microtubules, actin filaments, and intermediate filaments.
- The smooth endoplasmic reticulum functions in many metabolic processes. It synthesizes lipids, phospholipids as in plasma membranes, and steroids.
- The mitochondria produced energy/ATP
- Mitochondria have a double membrane arrangement that separates the organelle into four distinct compartments
- The cytosol contains an organized framework of fibrous molecules that constitute the cytoskeleton
Plss help me pass . I am so confused in chemistry !!

Answers
Answer:
Explanation:
Ok so an atom is each ball. So in the first one there are 5 balls. In the second one there are 4 and so on. A molecule contains more than two balls. So they are all molecules. For the counting reactants and products, count how many balls are to the left of the arrow which is your number of reactants and count the balls to the right to find the number of product atoms.
-. How many moles in 49.8 Liters of O2 at STP?
Answers
Answer:
2.22 mole
Explanation:
At STP one mole of an ideal gas occupies 22.4 L of volume.
Hence,
Volume = mol × 22.4 L/mol
solving for mole,
Mole = Volume / 22.4 L/mol
Putting values,
Mole = 49.8 L / 22.4 L/mol
Mole = 2.22 mole
Soil is only useful if scientists can match it to a location. true or false
Answers
It is false that soil is only useful if scientists can match it to a location.
How is soil important?Soil is the unconsolidated mineral or organic material on the immediate surface of the earth that serves as a natural medium for the growth of land plants.
The following are reasons why soil is an important substance in science;
Soil provides ecosystem services critical for lifeSoil acts as a water filter and a growing medium as it provides habitat for billions of organismsSoil contributes to biodiversity, which supplies most of the antibiotics used to fight diseases.Therefore, the location of a soil sample doesn't have to be known for the soil to useful to scientists.
Learn more about soil at: https://brainly.com/question/31227835
#SPJ1
Water is homogeneous substance true or false
Answers
Answer: pure water is homogeneous and pure substance.
Explanation: However, when a homogeneous substance consists of two or more different types of molecules uniformly intermingled with one another, then it’s called a homogeneous mixture. A mixture’s composition can vary, but a pure substance does not.
Answer:
True
Explanation:
since the gases and minerals dissolved in water are in the same state as water and they do not form separate layers