Answers
Fe2O3 + 3 C = 2 Fe + 3 CO is the reaction balanced equation. 3.79 L of CO are consequently produced.
If 2.90 moles of iron Fe are generated, how much carbon dioxide CO will be produced?where V denotes volume, w denotes iron weight, and h denotes carbon dioxide volume. Using the equation, we thus arrive at: V = 2.90 × 10-3 m3 Hence, 2.90 m3 of CO2 were created.
As a result, the amount of CO produced from the reaction of 4.00 moles of Fe2O3 can be computed as follows:
4.00 mol Fe2O3 × (3 mol CO / 1 mol Fe2O3) = 12.00 mol CO
So, 12.00 moles of CO are produced.
The ideal gas law can be used to convert moles of CO to volume at 745 torr and 23°C: PV = nRT, where R is the gas constant, n is the number of moles, P is the pressure, V is the volume, and T is the temperature in Kelvin.
The temperature must first be converted from Celsius to Kelvin:
23°C + 273.15 = 296.15 K
The optimum petrol rule can then be modified to account for volume:
V = (nRT) / P
Plugging in the values:
V = (12.00 mol × 0.08206 L·atm/K·mol × 296.15 K) / 745 torr
V = 3.79 L
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
Related Questions
If I have 1000ml of water and 10 ml of water, which will have the greater density? Why?
Answers
Answer:
1000 ml
Explanation:
hope it helpssssssss
Answer:
1000 ml because the bigger ml the greater density brainliest pls
Name the structure CH3coch2c(br)2ch2ch3
Answers
The name of the compound from the structure that we can see in the question is;
4,4-dibromohex-2one
Summary of how you name an organic compoundThe International Union of Pure and Applied Chemistry (IUPAC) established a systematic set of guidelines for naming organic compounds.
Find the compound's longest continuous chain of carbon atoms. The compound's name is derived from this chain, which also acts as the compound's parent chain.
Assign a number to each carbon atom in the parent chain to give each carbon atom in the compound a special identification. The end that is closest to the functional group or substitutes is where the numbering begins.
Learn more about organic compound:https://brainly.com/question/13508986
#SPJ1
Aluminum chloride is formed by reacting 13.34g aluminum with 52.82g chlorine. What is the percent composition of the compound?
Answers
I require assistance.

Answers
pH is defined as the negative logarithm (base 10) of the hydrogen ion concentration ([H+]) in a solution. Therefore, option (C) is correct.
In other words, the pH scale measures the acidity or basicity of a solution. The lower the pH value, the more acidic the solution is, and the higher the pH value, the more basic or alkaline the solution is.
For example, pure water has a pH of 7, which is considered neutral, meaning it is neither acidic nor basic. Acids have pH values lower than 7, while bases have pH values greater than 7. The pH scale is important in many areas of chemistry, including biochemistry, environmental science, and medicine.
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ1
"write the chemical equation between hydrogen (h) and carbonate (CO3) and the product(s)"
Answers
Answer:
On heating sodium hydrogen carbonate (NaHCO3). It decomposes to form sodium carbonate (Na2CO3), water (H2O) and carbon dioxide (CO2)
(ii) 2NaHCO3(s)Heatsodiumcarbonate(sodaash)Na2CO3(s)+CO2(g)+H2O(l)
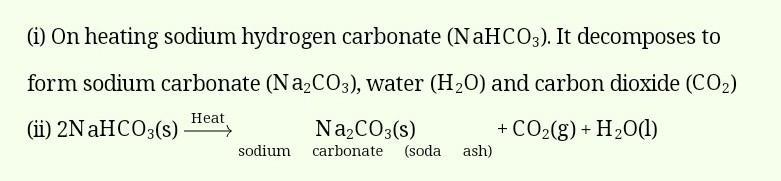
Answer:
Explanation:
2H + + CO3 2- = H2CO3
Determine the heat (q) required to raise the temperature of 80.0 grams of metal from 28.0 °C to 53.0 °C. Assume the specific heat of the material is 0.8 J/g°C. Be sure to use proper labels, fill in all blanks, and use complete sentences where applicable for full credit.
Heat (q) = ______________ J
Mass (m) = ____________ g
heat (s) = ______ J/g°C
Initial temp (T1) = ______ °C
Final temp (T2) = _______ °C
Is the process endothermic or exothermic? _______________________________
Answers
Answer:
Explanation:
m= 80.0 g
Cp=0.8 J/gc
Change in T= 25
q=Cp X m X T =0.8 X 80.0 X 25 =1600J
Postive q or enthalpy makes it endothermic.
How does the chemical structure of a substance affect its interaction with other substances?
Answers
This is due to the fact that a substance's chemical qualities, such as its molecular form, polarity, and functional groups, govern how it behaves and interacts with other substances.
How does their chemical makeup impact their chemical characteristics?By illustrating the spatial arrangement of atoms and chemical bonds within the molecule, chemical structure establishes the molecular geometry of a compound. In doing so, chemists are given a crucial visual depiction of a chemical formula.
In what ways do drugs interact with one another?In a chemical reaction, reactants come into contact with one another, atoms in the reactants break their connections with one another, and then the atoms reorganise and form new bonds to create the products.
To know more about molecule visit:-
https://brainly.com/question/19556990
#SPJ1
Can someone help me I only have 5 minutes left.

Answers
list the 3 pKa's for H3PO4
Answers
Answer:
The three pKa values for phosphoric acid (H3PO4) are 2.12, 7.21, and 12.32.
how do you jack hoff
Answers
An atom has the electron configuration shown below. How many valence electrons are in this atom?
Captionless Image
2
3
4
6
What charge would you expect on an ion of this fictitious atom?
Captionless Image
+3, cation
+5, cation
-5, anion
-3, anion
Which occurrence explains why noble gases are essentially unreactive?
Dipole Interactions
VSEPR Theory
Dispersion Forces
Octet Rule
A covalent bond occurs when ________ are shared between two atoms.
electrons
protons
neutrons
dipoles.
A substance conducts electricity well and has a boiling point of 1478 °C. Is this likely an ionic or covalent substance?
Not enough information to answer.
ionic
covalent
Select all the compounds listed below that are covalent compounds.
B
C
D
A
Captionless Image
A
B
C
D
Captionless Image
Trihocus Dipocus
Dihocus Tripocus
Hocus (II) Pocus
Hocus (III) Pocus
Two nonmetal Halloween elements react to form the compound TriTrick DiTreat. What is the formula? Trick = X and Treat = Y
C
D
B
A
Write the formula for Candycorn Pumpkinate. Candycorn (Cc) has a charge of +3 and pumpkinate (PkO) a charge of -2.
A
B
C
D
Which statement best describes the atomic structure of metals?
Valence electrons packed in a lattice arrangement around cations.
Valence electrons packed in a lattice arrangement around anions.
Closely packed cations with loosely held valence electrons.
Loosely packed cations with tightly held valence electrons.
Which of the following properties is NOT explained by metallic bonding?
Crystalline structures
Malleabilty
Ductility
Thermal conductivity
What is the ionic charge of element X in the compound XP? Phosphorus is in group 15 on the periodic table.
1+
1-
3+
3-
_____________is not reactive with only 2 electrons in its valence shell.
Krypton
Helium
Neon
Xenon
How many electrons are shared in the double bonds in the structural formula shown below?
Captionless Image
2
4
6
8
Option 5
How many lone PAIRS are shown in the molecule below?
Captionless Image
none
2
4
8
A molecule of XY (2 nonmetals) is polar because
X is a metal so exhibits magnetic properties.
X and Y are both ions.
it is ionic and all ionic bonds are polar.
the X atom is able to attract the shared electrons more strongly than a Y atom.
Answers
Answer:
question no 4 answer is electrons
1. What process occurs along divergent plate boundary?
2. What geologic features formed when two plates moved away from each other?
3. Compare rift valley from mid-ocean ridge. Explain your answer. PIVOT 4A CALABARZON 24
Answers
1. Earthquakes and Eruptions (volcanoes)
2. Divergent plate boundary
3. Apart from a widening valley, it equally contributes to steep mountain sides spreading. A mid-oceanic ridge is naturally produced if two oceanic plates naturally start moving away from each other.
Explanation:1. When a divergent boundary occurs beneath oceanic lithosphere, the rising convection current below lifts the lithosphere, producing a mid-ocean ridge. Extensional forces stretch the lithosphere and produce a deep fissure. When the fissure opens, pressure is reduced on the super-heated mantle material below. It responds by melting, and the new magma flows into the fissure. The magma then solidifies and the process repeats itself.
2. When two plate diverge from each other also known as divergent (di-apart, vergere- to inclined) plate margin .
Two plate diverge along oceanic ridges where new lithosphere is created.
Now come to the main point: Geological Features
When two oceanic plate diverge then new sea floor is created by a process called sea floor spreading and features like Mid oceanic ridges, volcanoes & Young lava flows forms. Ex. East Pacific Rise, Mid-Adlantic Ridge.
When two continental plates diverge by continental rifting then features like Rift valley, volcanoes are found. Ex of continent continent -East Africa rift vally, Red sea.
3. The major difference between different types is what type of plate the divergent boundary is between. If the boundary is found between two continental plates you are left with a rift valley. ... If two oceanic plates begin moving away from each other it creates a mid-oceanic ridge.
Thank You!
Please Mark Me Brainliest!
Have a good day studying!
What are some convincing reasons for researcher's to choose your biome ( P.S. this is for a project) pls reply as soon as you can
Answers
Choosing a biome for a research project can provide a wealth of opportunities to better understand the natural world and contribute to important conservation and management efforts.
Why is biome important in research?There are several convincing reasons for researchers to choose a particular biome for their project, some of which include:
Biodiversity: Biomes are characterized by their unique plant and animal species, which can provide a diverse range of research opportunities, including studying their adaptations, behaviors, and interactions.
Climate Change: Biomes are also influenced by climate change, making them an important area of research to better understand how these ecosystems are being affected by changes in temperature and precipitation patterns.
Human Impacts: Human activities, such as deforestation, agriculture, and urbanization, can have a significant impact on biomes. Studying these impacts can help researchers better understand how to mitigate and manage these effects.
Biogeochemical Cycles: Biomes are also important for studying biogeochemical cycles, such as the carbon and nitrogen cycles, which are essential for sustaining life on Earth.
Conservation: Finally, studying biomes can also contribute to conservation efforts, helping to preserve these unique ecosystems and the species that inhabit them.
Find out more on biome here: https://brainly.com/question/2096327
#SPJ1
Which H₂S molecule's dipole moment is correctly labeled?
Help

Answers
We know that the dipole moment moves from the negative to the positive line thus it is shown by option A
What is the dipole moment?The dipole moment is a measure of the polarity of a chemical bond or a molecule. It is defined as the product of the magnitude of the charge on either end of a polar covalent bond and the distance between them. The dipole moment of a molecule is a vector quantity, with a direction pointing from the negatively charged end of the molecule to the positively charged end.
In simple terms, the dipole moment represents the separation of positive and negative charges within a molecule. The greater the dipole moment of a molecule, the more polar it is and the more likely it is to interact with other polar molecules or ions.
Learn more about dipole moment:https://brainly.com/question/14140953
#SPJ1
Please help me with this
If somebody posts b.u.l.l.s.h.i.t. answers, please report them!!
Answers
Answer:
where is the question
Explanation:
who is th father of chemistry
Answers
You just finished your favorite soda which contained 39.0 grams of sugar (C12H22O11). How many molecules of sugar did you ingest?
Answers
The number of molecules of sugar, C₁₂H₂₂O₁₁ you ingest given the data is 6.86×10²² molecules
Avogadro's hypothesis1 mole of C₁₂H₂₂O₁₁ = 6.02×10²³ molecules
But
1 mole of C₁₂H₂₂O₁₁ = 342 g
Thus,
342 g of C₁₂H₂₂O₁₁ = 6.02×10²³ molecules
How to determine the molecules in 39 g of sugar342 g of C₁₂H₂₂O₁₁ = 6.02×10²³ molecules
Therefore,
39 g of C₁₂H₂₂O₁₁ = (39 × 6.02×10²³) / 342
39 g of C₁₂H₂₂O₁₁ = 6.86×10²² molecules
Thus, 6.86×10²² molecules are present in 39 g of sugar, C₁₂H₂₂O₁₁
Learn more about Avogadro's number:
https://brainly.com/question/26141731
#SPJ1
How many liters of ethylene glycol antifreeze (C 2H 6O 2) would you add to your car radiator containing 15.0 L of water if you needed to protect your engine to –17.8°C? (The density of ethylene glycol is 1.1 g/mL. For water, K f = 1.86°C/m.)
Answers
Answer:
\(V=8.10L\)
Explanation:
Hello,
In this case, we can use the freezing point depression formula in order to compute the molality of the mentioned solute (ethylene glycol) considering a van't hoff factor of 1 since it is a covalent molecule:
\(\Delta T=-i*m*Kf\\\\m=\frac{\Delta T}{i*Kf}=\frac{(-17.8-0)\°C}{1*1.86\°C/m}=9.57m\)
Next, since water density is 1 kg/L and molal units are mol/kg, we compute the present moles of solute:
\(n=9.57mol/kg*15.0kg=143.55mol\)
Next, the mass with its molar mass (62.07 g/mol):
\(m=143.55mol*62.07g/mol=8910.05g\)
Finally, with the given density we compute the required volume in liters:
\(V=8910.05g*\frac{1mL}{1.1g} *\frac{1L}{1000mL}\\ \\V=8.10L\)
Best regards.
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Richard sketched models of two different neutral isotopes of oxygen. If the
isotope models are accurate, which statement might be true? (Refer to the
periodic table if necessary.)
A. One shows 7 protons, and one shows 9.
B. One shows 8 neutrons, and one shows 9.
C. One shows 8 electrons, and one shows 9.
D. One shows 8 protons, and one shows 7.
Answers
Answer:
its b
Explanation:
just did the test on A P E X
If the isotope models are accurate, One shows 8 neutrons, and one shows 9. Therefore, the correct option is option B.
What is isotope?A chemical element's isotope is one of more than one species of atoms that share the same atomic number, spot on the periodic table, and almost identical chemical activity, but differ in atomic mass and physical characteristics. There are a number of isotopes for each chemical element.
The first step in identifying and labelling an atom is to count the protons within its nucleus. Usually, this nuclear number is denoted by the letter Z. The fact that all atoms possessing the same number of electrons have essentially equal chemical characteristics lends the atomic number its enormous significance. If the isotope models are accurate, One shows 8 neutrons, and one shows 9.
Therefore, the correct option is option B. If the isotope models are accurate, One shows 8 neutrons, and one shows 9.
To know more about isotope, here:
https://brainly.com/question/11680817
#SPJ5
match each substance correctly to the principal type(s) of intermolecular force(s) present, other than covalent bonding.
Answers
Substance intermolecular force
CH2OH ---> Hydrogen bonding
CH3F --> Dipole-dipole forces
C3H8 --> Dispersion forces
CaCL2 --> Ionic bonding
The intermolecular force present in CH2OH is hydrogen bonding. The intermolecular force present in CH3F is Dipole-dipole forces. Ionic bonding is defined as a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities. It is the primary interaction occurring in ionic compounds. Hydrogen bonding results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
Dipole-dipole forces are defined as a attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. Dispersion force is defined as a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.
To learn more about Intermolecular forces
https://brainly.com/question/12243368
#SPJ4
The complete question is,
Match each substance correctly to the principal type(s) of intermolecular force(s) present, other than covalent bonding.
CH2OH Ionic bonding
CH3F Hydrogen bonding
C3H8 Dispersion forces
CaCL2 Dipole-dipole forces
What is the oxidation state of N in NaNOz?

Answers
The oxidation state of nitrogen (N) in NaNO3 is +5. option B
To determine the oxidation state of nitrogen (N) in sodium nitrate (NaNO3), we need to assign oxidation numbers to each element in the compound.
In NaNO3, we know that the sodium ion (Na+) has a +1 oxidation state because it is an alkali metal. Oxygen (O) typically has an oxidation state of -2 in compounds, and there are three oxygen atoms in NaNO3. Since the compound is neutral, the sum of the oxidation states must be zero.
Let's assume that the oxidation state of nitrogen is x. Therefore, we can set up the equation:
(+1) + x + (-2) * 3 = 0
Simplifying the equation:
+1 + x - 6 = 0
x - 5 = 0
x = +5
Therefore, the oxidation state of nitrogen (N) in NaNO3 is +5.
The oxidation state of an element indicates the number of electrons it has gained or lost in a compound. In this case, the nitrogen atom in NaNO3 has gained five electrons to achieve a stable oxidation state of +5.
It is important to note that oxidation states are formal charges and do not necessarily represent the actual distribution of electrons in a compound. They are assigned based on a set of rules and can be useful in understanding the reactivity and behavior of elements in chemical reactions.
Option B
For more such questions on oxidation state visit:
https://brainly.com/question/25551544
#SPJ8
pls help will give brainilest or however you spell it

Answers
Answer:
1. balanced
2. balanced
3. unbalanced
4. balanced
5. balanced
6. unbalanced
7. balanced
8. unbalanced
Explanation:
oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
WHATS THE LAST OPTION HELP ASAP I WILL MARK BRAINLIEST

Answers
Answer:
SI-28
Explanation:
Explain how these results show that chlorine is more reactive than bromine and
lodine.
Answers
Chlorine is more reactive than bromine because it replaces both bromine and iodine.
How chlorine is more reactive than bromine?Fluorine is the most sensitive while on the other hand, the astatine is the least reactive as compared to other elements. The chlorine displaces both bromine and iodine, and bromine displaces iodine because of its high reactivity. The element that replaces other atom is considered as more reactive.
The order of reactivity is that the chlorine is more reactive than bromine, which indicates that chlorine is more reactive than iodine.
So we can conclude that chlorine is more reactive than bromine due to high reactivity.
Learn more about Chlorine here: https://brainly.com/question/24218286
#SPJ1
why do you continuously gain exactly the amount of mass you consume with each meal
Answers
Answer:
It depends on how much the calories and fat there is in the meals and if you don't get enough physical activity of the same amount of food that you eat it can lead to weight gain
Explanation:
When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Complete the sentences to best explain the difference. Match the words to the appropriate blanks in the sentences. Make certain each sentence is complete before submitting your answer.
1. The strongest force observed at the surface of glass is:________
2. Water is___________ and interacts, generating adhesive interactions with the only weak dispersion strong hydrogen bonding polar glass
3. Hexane is________ and interacts, generating____________ adhesive interactions with the glass.
a. dipole
b. nonpolar
c. only weak
d. dispersion
e. strong
f. hydrogen bonding
g. polar
Answers
Answer:
Explanation:
1 . The strongest force observed at the surface of glass is:__DIPOLE______
2. . Water is__POLAR_________ and interacts, generating adhesive interactions with the only weak dispersion strong hydrogen bonding polar glass.
3 . Hexane is_NON-POLAR _______ and interacts, generating__ONLY WEAK __________ adhesive interactions with the glass.
Which of these is a likely impact of the stronger than normal trade winds on the eastern Pacific ocean?
Warm surface water builds up, causing lower than average temperature.
Warm surface water builds up, causing higher than average temperature.
Warm surface water is reduced, causing colder conditions than normal.
Warm surface water is reduced, causing hotter conditions than normal.
Answers
Answer:
the answer is c I tink good luck
Answer:
C. Warm surface water is reduced, causing colder conditions than normal.
Explanation:
During El Niño, trade winds are weak. During La Niña, it's the opposite. The surface winds across the entire tropical Pacific are stronger than usual, and most of the tropical Pacific Ocean is cooler than average. Rainfall increases over Indonesia (where waters remain warm) and decreases over the central tropical Pacific
how to synthesize tripropylamine from propylene
Answers
The reactions that result in the emission of light involve the ruthenium label and tripropylamine (TPA), two electrochemically active molecules.
Thus, The electrode surface inside the measurement cell is where the reactions take place.
The ruthenium label is oxidized at the electrode surface as an electrical potential is applied, and TPA is oxidized into a radical cation that spontaneously loses a proton.
When the resultant TPA radical interacts with oxidized ruthenium, the ruthenium label enters an excited state and emits a photon (620 nm) before decaying. The ruthenium label is renewed and ready to carry out numerous light-generating cycles as it goes back to its ground state.
Thus, The reactions that result in the emission of light involve the ruthenium label and tripropylamine (TPA), two electrochemically active molecules.
Learn more about tripropylamine, refer to the link:
https://brainly.com/question/32070784?
#SPJ1