Using the periodic table above and your knowledge of patterns and trends on the table, which of the following elements is the best conductor of electricity? (1 pt)
*
1 point
Titanium (Ti, #22)
Silicon (Si, #14)
Oxygen (O, #8)
Argon (Ar, #18)
Answers
The metals are good conductor of electricity, thus among this titanium is the best conductor of electricity.
What are conductors?Conductors is defined as a substance or type of material that allow flow of charge or electricity in one or more direction. Metals are commonly consider as a good conductor of electricity. It allow electricity to flow through them very easily and produce electric current.
Metals are defined as the elements that are typically hard due to presence of strong metallic connection between the atoms.
Thus, metals are good conductor of electricity, thus among this titanium is the best conductor of electricity.
To learn more about conductors, refer to the link below:
https://brainly.com/question/18084972
#SPJ1
Related Questions
Name the following alkane molecule:
CH3CH(CH3)CH2CH(CH3)2
A. heptane
B. 2,4,4-trimethylbutane
C. 2,4-dimethylpentane

Answers
Answer:
B
Explanation:
Just by looking at the amount of carbons on the straight chain which is four and whom prefix is butane we can see that B is the answers
If you wanted to heat a liquid that would splatter when it is heated, you would heat it in a
Answers
Answer:
This question lacks options but it will be answered based on the fact that laboratory processes including heating are done using glasswares. The answer is:
Conical or Erlenmeyer/Boiling flask
Explanation:
Glasswares are simply laboratory equipments made of glass. They are used in performing the various laboratory processes like mixing, storing chemicals, heating and cooling etc. Glasswares includes flasks e.g volumetric flask, Erlenmeyer flask etc, beaker, test tube, graduated cylinder etc.
Heating a liquid that would splatter or splash in a laboratory requires using a glassware that can prevent spilling of the liquid outside it. A FLASK such as Erlenmeyer or boiling flask is best suited for this purpose because they generally have a conical shaped body with a narrow neck that prevents liquid chemicals from spilling when heated or shaken. Flasks can also be corked with a lid to prevent the liquid from coming out.
briefly summarize the procedures of an autopsy. explain which of those procedures help in establishing cause of death, manner of death, and postmortem interval and how
Answers
An autopsy is a medical procedure that is conducted to determine the cause of death. It is performed by a medical examiner, who may be a doctor, a medical scientist, or other qualified personnel.
Here, correct answer will be
The autopsy procedure usually involves the examination of the body, including the internal organs and tissues. Samples of the organs and tissues may be taken for further analysis and microscopic examination.
The examiner will also review the medical history and any notes from the doctor or hospital staff. All of these procedures help in establishing the cause of death, the manner of death, and the postmortem interval. The cause of death is determined by the examination of the body, which may reveal signs of trauma or disease.
The manner of death may be determined by looking at the circumstances surrounding the death, such as the presence of drugs or alcohol in the body or the presence of any weapons. The postmortem interval is determined by looking at the body's decomposition and other evidence, such as insect activity.
Know more about autopsy here
https://brainly.com/question/6463046#
#SPJ11
1. Explain the relationship
between Polaris and Earth's tilt
Answers
Answer:
that it i think
Explanation:
the earth revolves around the Sun once each year and spins on its axis of rotation once each day. This axis of rotation is tilted 23.5 degrees relative to its plane of orbit around the Sun. The axis of rotation is pointed toward Polaris the North Star. As the Earth orbits the Sun the tilt of Earth’s axis stays lined up with the North Star.
If an 83.00 g sample of Iron has a starting temperature of 297K and an ending temperature of 329K, how much heat will be lost from the iron sample?
Answers
Answer:
1192.54 J
Explanation:
Applying,
Q = cm(t₂-t₁)................ Equation 1
Where Q = Amount of heat lost, c = specific heat capacity of the iron, m = mass of the iron, t₁ = initial temperature, t₂ = final temperature.
From the question,
Given: m = 83 g = 0.083 kg, t₁ = 297 K, t₂ = 329 K
Constant: c = 449 J/kg.K
Substitute these values into equation 1
Q = 449(0.083)(329-297)
Q = 449(0.083)(32)
Q = 1192.54 J
In our chem lab. We have to do something called the chem 21. This weeks topic was about solving ten test tube mystery and basically we were using a plate to mix it by one chemical to one chemical.We discovered thatA=KlB=Na2sC=NH4ClD=HClE=AgNo3F=BaCl2G=H2SO4H=NaBrI=NaOHJ=CuSO4However, we have to explain why and write the net ionic equation?
Answers
our chemistry lab. The chem 21 is a requirement for us. Basically, we were using a plate to chem lab it by one chemical to one.
Your Instructor has defined tolerances, which the Chem21 computer uses to score your response. This week's theme was about solving ten test tube mysteries. You will be informed on the website whether you are required to round your response chem lab to the correct number of Significant Figures; otherwise, you need just enter a number that lies between the correct amount minus
Here are some Chemistry Practical Class 12 experiments that will help students effectively prepare for their exams. I Making potash alum or ferrous ammonium sulphate double salt. Identifying a cation and an anion in a.
To learn more about chem lab please click on below link
https://brainly.com/question/28447712
#SPJ4
Choose the electron transition which will absorb the photon of smallest ν.
n = 1 to n = 3
n = 4 to n = 5
n = 6 to n = 5
n = 4 to n = 2
n = 4 to n = 3
Answers
The electron transition that will absorb the photon of smallest frequency (ν) is n = 6 to n = 5, as it involves the smallest energy difference between the energy levels.
When an electron in an atom absorbs a photon, it moves to a higher energy level or orbital. The energy of the photon must match the energy difference between the initial and final energy levels. The frequency (ν) of the photon is directly proportional to its energy (E), and inversely proportional to its wavelength (λ). The electron transition that absorbs the photon of smallest frequency involves the smallest energy difference between the energy levels, which is n = 6 to n = 5. This means that the wavelength of the absorbed photon will be relatively longer compared to the other transitions listed. Understanding these electron transitions is important for many applications, such as spectroscopy, lasers, and quantum computing.
Learn more about electron transition here:
https://brainly.com/question/18156550
#SPJ11
now calculate the theoretical percent hydrolysis for these 1m solutions. 1 M NaC2H3O2_______. 1 M Na2CO3_________.
Answers
To calculate the theoretical percent hydrolysis for the given 1 M solutions, we need to consider the hydrolysis reactions of the respective salts. Therefore, the theoretical percent hydrolysis for both 1 M NaC2H3O2 and 1 M Na2CO3 solutions is 100%.
For 1 M NaC2H3O2 (sodium acetate):
The hydrolysis reaction is as follows:
CH3CO2^- + H2O ⇌ CH3COOH + OH^-
Theoretical percent hydrolysis can be calculated using the equation:
Percent hydrolysis = [OH-] / initial concentration of salt × 100
Since NaC2H3O2 is a strong electrolyte, it completely ionizes in water, giving 1 M of CH3CO2^- ions.
Thus, [OH-] = 1 M
Percent hydrolysis = 1 M / 1 M × 100
= 100%
For 1 M Na2CO3 (sodium carbonate):
The hydrolysis reaction is as follows:
CO3^2- + 2H2O ⇌ HCO3^- + OH^-
Similar to the previous calculation, since Na2CO3 is a strong electrolyte, it completely ionizes in water, providing 1 M of CO3^2- ions.
Thus, [OH-] = 1 M
Percent hydrolysis = 1 M / 1 M × 100
= 100%
To know more about hydrolysis
https://brainly.com/question/30468294
#SPJ11
a crystal has an enthalpy of formation for vacancies of 1.5 eV establishing a certain equilibrium concentration of vacancies at the temperature 1200 K by how much doe sthe temperature have to be raised to increase the vacancy concentration factor by 10
Answers
The temperature of the crystal have to be raised by approximately 261 K to increase the vacancy concentration factor by a factor of 10.
Enthalpy of formation for vacancies refers to the amount of energy required to create a vacancy in a crystal lattice. In this case, the crystal has an enthalpy of formation for vacancies of 1.5 eV, which establishes a certain equilibrium concentration of vacancies at the temperature of 1200 K.
To increase the vacancy concentration factor by 10, we need to raise the temperature of the crystal. The vacancy concentration factor is related to the equilibrium concentration of vacancies and the temperature according to the following equation:
K = Nv/N
Where K is the vacancy concentration factor, Nv is the number of vacancies, and N is the total number of lattice sites. At equilibrium, K is constant and depends only on the enthalpy of formation for vacancies and the temperature.
To increase K by a factor of 10, we need to increase the temperature by a certain amount. The relationship between K and temperature is given by the following equation:
K = exp(-Qv/kT)
Where Qv is the enthalpy of formation for vacancies, k is Boltzmann's constant, and T is the temperature in Kelvin. Taking the natural logarithm of both sides, we can solve for the temperature required to increase K by a factor of 10:
ln(K2/K1) = Qv/k * (1/T1 - 1/T2)
Where K1 is the initial value of K, K2 is the final value of K, and T1 is the initial temperature. Rearranging this equation and substituting the given values, we get:
T2 = Qv/k * (ln(K2/K1)/10 + 1/T1)
Plugging in the values for Qv (1.5 eV), k (8.617 x 10^-5 eV/K), K1 (the equilibrium value at 1200 K), K2 (10 times the equilibrium value), and T1 (1200 K), we get:
T2 = 1461 K
Therefore, we need to raise the temperature of the crystal by approximately 261 K to increase the vacancy concentration factor by a factor of 10.
To know more about Enthalpy of vacancy formation, refer to the link below:
https://brainly.com/question/31683406#
#SPJ11
Not a timed or graded assignment. Quick answering will get an amazing review, thank you :)

Answers
This law states that matter can neither be created nor destroyed in an ordinary chemical reaction, but can change from one form to another.
What this implies is that when two elements or compound combine as a reactant to form a product, there's no loss of matter and the mass of the reactants must be equal to the mass of the product.
Given that
AlBr₃ + K₂SO₄ → KBr + Al₂(SO₄)₃
If we look closely at the above reaction, we would see that aluminuim is not balanced, potassium is also not balanced, bromine is not balance as well as sulphur and oxygen.
Let's put two moles attached to the AlBr₂ and three moles of K₂SO₄
2AlBr₃ + 3K₂SO₄ →
This would give us
6KBr + Al₂(SO₄)₃
If we add the two equations together,
2AlBr₃ + 3K₂SO₄ → 6KBr + Al₂(SO₄)₃
From the above, we have
2 atoms of Al on the reactant side and 2 atoms of Al on the product side
6 atoms of Br on the reactant side and 6 atoms of Br on the product side
6 atoms of K on the reactant side and 6 atoms of K on the product side
3 atoms of S on the reactant side and 3 atoms of S on the product side
12 atoms of O on the reactant side and 12 atoms of O on the product side
AS Chemistry Unit 1.3: Mole Calculations (Moles & Mass)
Relative Molecular Mass
1. Calculate the relative molecular mass of: a) H₂SO4
40-11(14.0x2)+(6x18
b) Ca(NO3)2 c) CuSO4.7H₂O
(2x1) + 32.1 + (16x 4) = 98.1
2. An oxide of nitrogen contains only one nitrogen atom per molecule and has a relative
molecular mass= 30. Determine its molecular formula. NO
3. An oxide of nitrogen contains only one oxygen atom per molecule and has a relative
molecular mass= 44. Determine its molecular formula.
N₂O
4. An oxide of sulfur contains only one sulfur atom per molecule and has a relative molecular
mass=80. Determine its molecular formula.
SO₂
5. An oxide of sulfur contains only one sulfur atom per molecule and has a relative molecular
mass=64. Determine its molecular formula.
Sx Oy
6. An oxide of silicon contains only one Si atom per molecule and has molar mass = 60 gmot¹.
Determine its molecular formula.
Calculating Moles, Mass & Molar Mass
1. Calculate the number of moles of atoms contained in 8g of Argon gas Ar. = m
Mr
=
7. Xenon forms a fluoride XeFx with molar mass = 169 gmol-¹. Determine the value of x in the
formula of xenon fluoride.
7 xe fz
(SiO₂
2. Calculate the number of moles of molecules contained in 1.25kg of H₂SO4.
1250
3. Calculate the mass of 0.04 moles of Ca(OH)2.
12-7 moles (2 + 32 +64) = 98
2.9g
4. Calculate the mass of 0.001 moles of copper sulphate pentahydrate CuSO4.5H₂O.
164-1
O.S
8
40.0
= 0.2
moles.
0.250 9
5. 0.2 moles of a hydrocarbon has a mass of 6g. Calculate the molar mass of the hydrocarbon.
30 gmol"!
6. 2.5 moles of an oxide of nitrogen has a mass of 115g. Calculate its molar mass.
115
Mole Ratio in Compound Formulae
= 46
gmai
1. a) How many moles of hydrogen atoms are present in 0.5 moles of HCI?
H-O-H=1
b) How many moles of hydrogen atoms are present in 0.5 moles of H₂O?
c) How many moles of oxygen atoms are present in 0.2 moles of Na2CO3?.
d) How many moles of chloride ions are present in 1.2 moles of MgCl₂? 2 x 2 = 2.4
mole.
0.5 +0.5 1
H-CI
O.S
Answers
1. The molar mass of a compound is the sum of the atomic masses of all the atoms present in one molecule of the compound. To calculate the relative molecular mass (also called the molecular weight or molar mass) of a compound, we add up the atomic masses of all the atoms in the compound.
1a) H₂SO₄
The atomic masses of H, S, and O are 1.008, 32.06, and 16.00, respectively.
Relative molecular mass of H₂SO₄ = (2 x atomic mass of H) + atomic mass of S + (4 x atomic mass of O)
= (2 x 1.008) + 32.06 + (4 x 16.00)
= 98.08
1b) Ca(NO₃)₂
The atomic masses of Ca, N, and O are 40.08, 14.01, and 16.00, respectively.
Relative molecular mass of Ca(NO₃)₂ = atomic mass of Ca + (2 x atomic mass of N) + (6 x atomic mass of O)
= 40.08 + (2 x 14.01) + (6 x 16.00)
= 164.09
1c) CuSO₄.7H₂O
The atomic masses of Cu, S, O, and H are 63.55, 32.06, 16.00, and 1.008, respectively.
Relative molecular mass of CuSO₄.7H₂O = atomic mass of Cu + atomic mass of S + (4 x atomic mass of O) + (14 x atomic mass of H)
= 63.55 + 32.06 + (4 x 16.00) + (14 x 1.008)
= 249.69
2. An oxide of nitrogen contains only one nitrogen atom per molecule and has a relative molecular mass = 30. Determine its molecular formula.
The relative molecular mass of the oxide of nitrogen (NOx) is 30.
The atomic mass of N is 14.01, and the atomic mass of O is 16.00.
We can set up the equation: 14.01 + 16.00x = 30
Solving for x, we get x = 1.
Therefore, the molecular formula of the oxide of nitrogen is NO.
3. An oxide of nitrogen contains only one oxygen atom per molecule and has a relative molecular mass = 44. Determine its molecular formula.
The relative molecular mass of the oxide of nitrogen (NOx) is 44.
The atomic mass of N is 14.01, and the atomic mass of O is 16.00.
We can set up the equation: 14.01x + 16.00 = 44
Solving for x, we get x = 2.
Therefore, the molecular formula of the oxide of nitrogen is N₂O.
4. An oxide of sulfur contains only one sulfur atom per molecule and has a relative molecular mass = 80. Determine its molecular formula.
The relative molecular mass of the oxide of sulfur (SOx) is 80.
5. The atomic mass of S is 32.06, and the atomic mass of O is 16.00.
We can set up the equation: 32.06 + 16.00x = 80
Solving for x, we get x = 3.
Therefore, the molecular formula of the oxide of sulfur is SO₃.
6. The molar mass of the oxide of silicon (SiOx) is 60 g/mol.
The atomic mass of Si is 28.09, and the atomic mass of O is 16.00.
We can set up the equation: 28.09 + 16.00x = 60
Solving for x, we get x = 2.
Therefore, the molecular formula of the oxide of silicon is SiO₂.
1. The molar mass of Ar is 39.95 g/mol. Therefore, the number of moles of Ar in 8 g is:
moles = mass / molar mass = 8 g / 39.95 g/mol = 0.200 moles
2. The molar mass of H₂SO₄ is 98.08 g/mol (2 + 32 + 32 + 4x16). Therefore, the number of moles of H₂SO₄ in 1.25 kg is:
moles = mass / molar mass = 1250 g / 98.08 g/mol = 12.73 moles
3. The molar mass of Ca(OH)₂ is 74.09 g/mol (40.08 + 2x1.01 + 2x16.00). Therefore, the mass of 0.04 moles of Ca(OH)₂ is:
mass = moles x molar mass = 0.04 moles x 74.09 g/mol = 2.96 g
4. The molar mass of CuSO₄·5H₂O is 249.68 g/mol (63.55 + 32.07 + 4x16.00 + 5x18.02). Therefore, the mass of 0.001 moles of CuSO₄·5H₂O is:
mass = moles x molar mass = 0.001 moles x 249.68 g/mol = 0.250 g
5. The mass of the hydrocarbon is given as 6 g, and the number of moles is 0.2 moles. Therefore, the molar mass of the hydrocarbon is:
molar mass = mass / moles = 6 g / 0.2 moles = 30 g/mol
6. The mass of the oxide of nitrogen is given as 115 g, and the number of moles is 2.5 moles. Therefore, the molar mass of the oxide of nitrogen is:
molar mass = mass / moles = 115 g / 2.5 moles = 46 g/mol.
1.a) In 1 molecule of HCl, there is 1 hydrogen atom. Therefore, in 0.5 moles of HCl, there are:
0.5 moles HCl x 1 mole H per 1 mole HCl = 0.5 moles H
b) In 1 molecule of H₂O, there are 2 hydrogen atoms. Therefore, in 0.5 moles of H₂O, there are:
0.5 moles H₂O x 2 moles H per 1 mole H₂O = 1 mole H
c) In 1 molecule of Na₂CO₃, there are 3 oxygen atoms. Therefore, in 0.2 moles of Na₂CO₃, there are:
0.2 moles Na₂CO₃ x 3 moles O per 1 mole Na₂CO₃ = 0.6 moles O
d) In 1 molecule of MgCl₂, there are 2 chloride ions. Therefore, in 1.2 moles of MgCl₂, there are:
1.2 moles MgCl₂ x 2 moles Cl⁻ per 1 mole MgCl₂ = 2.4 moles Cl⁻
A sample of hydrogen is collected in a flask over water. The partial pressures are 789 mmHg for H₂
and 60 mmHg for H₂O. What is the total pressure exerted by the mixture of gasses?
Answers
The total pressure of the gas is calculated as 849 mmHg.
What is partial pressure?The term partial pressure is the pressure that is exerted by an individual gas in a mixture of gases.
In this case, the partial pressures of hydrogen and water are 789 mmHg and 60 mmHg respectively.
Thus, the total pressure of the gas = 60 mmHg + 789 mmHg = 849 mmHg.
Learn more about partial pressure:https://brainly.com/question/14281129
#SPJ1
Unsaturated fatty acids are susceptible to damage by exposure to light, oxygen, or heat. This breakdown of fatty acids may cause disagreeable flavors and odors in food products. A fatty acid that has been damaged in this way is
Multiple Choice
rancid.
desaturated.
emulsified.
hydrogenated.
Answers
Unsaturated fatty acids are susceptible to damage by exposure to light, oxygen, or heat. This breakdown of fatty acids may cause disagreeable flavors and odors in food products. A fatty acid that has been damaged in this way is rancid. Thus, the correct option is A.
What is Rancidity?The term 'rancid' refers to a state where an edible fat or oil has become stale, producing an unpleasant odor or flavor. When exposed to light, oxygen, or heat, unsaturated fatty acids, in particular, are susceptible to rancidity. Rancidity occurs due to the oxidation of the fatty acids present in the food products. This process leads to a breakdown of the fatty acids, causing disagreeable flavors and odors in the food products.
As such, when food products are stored, it is essential to minimize the exposure of unsaturated fatty acids to light, oxygen, or heat to avoid rancidity.
Therefore, the correct option is A.
Learn more about Rancidity here:
https://brainly.com/question/31179477
#SPJ11
Calculate the difference in binding energy per nucleon for the isobars 23/11 Na (23 being the mass number and 11 being atomic number) and 23/12 Mg.
Answers
The difference in binding energy per nucleon between 23/11 Na and 23/12 Mg can be calculated by finding the total binding energy for each isobar and dividing it by the respective number of nucleons.
To calculate the difference in binding energy per nucleon between the isobars 23/11 Na and 23/12 Mg, we need to find the total binding energy for each isobar and then divide it by the respective number of nucleons.
The atomic mass of 23/11 Na is 23, which means it has 23 nucleons (protons and neutrons). The atomic number is 11, indicating it has 11 protons.
The atomic mass of 23/12 Mg is also 23, so it has 23 nucleons. However, the atomic number is 12, indicating it has 12 protons.
We can use the equation:
Binding Energy per Nucleon = (Total Binding Energy) / (Number of Nucleons)
To find the total binding energy, we can consult a table or use an approximate average value. Let's assume the average binding energy per nucleon for both elements is 8.5 MeV (million electron volts).
For 23/11 Na:
Binding Energy per Nucleon = (Total Binding Energy of Na) / (Number of Nucleons)
= (8.5 MeV) / (23 nucleons)
For 23/12 Mg:
Binding Energy per Nucleon = (Total Binding Energy of Mg) / (Number of Nucleons)
= (8.5 MeV) / (23 nucleons)
The difference in binding energy per nucleon can then be calculated by subtracting the value for Na from the value for Mg.
learn more about binding energy here:
https://brainly.com/question/32197843
#SPJ11
With respect to solubility equilibria (ksp), what are the key differences in pb2 solubility with and without orthophosphate added? how would the concentration of free pb2 be expected to change?
Answers
The addition of orthophosphate ions (PO43-) decreases the solubility of Pb2+ ions by forming insoluble lead orthophosphate (Pb3(PO4)2). This reduces the concentration of free Pb2+ ions in solution. Without orthophosphate, Pb2+ ions remain more soluble and are present as free ions in higher concentrations.
When orthophosphate (PO43-) is added to a solution containing lead ions (Pb2+), it forms an insoluble compound called lead orthophosphate (Pb3(PO4)2). The addition of orthophosphate affects the solubility of lead ions, leading to key differences in the solubility equilibrium and the concentration of free Pb2+.
Without orthophosphate:
In the absence of orthophosphate, the solubility equilibrium of lead compounds, such as lead chloride (PbCl2) or lead nitrate (Pb(NO3)2), can be represented as follows:
PbX2 (s) ⇌ Pb2+ (aq) + 2X- (aq)
The solubility product constant (Ksp) expression for this equilibrium is given by:
Ksp = [Pb2+] [X-]^2
Where [Pb2+] represents the concentration of free lead ions and [X-] represents the concentration of the anion derived from the lead compound.
With orthophosphate:
Upon adding orthophosphate (PO43-), the following reaction occurs between lead ions and orthophosphate ions:
Pb2+ (aq) + 2PO43- (aq) ⇌ Pb3(PO4)2 (s)
Lead orthophosphate (Pb3(PO4)2) is insoluble and precipitates out of the solution. This removal of lead ions from the aqueous phase effectively reduces the concentration of free Pb2+ in the solution.
As a result, the concentration of free Pb2+ decreases in the presence of orthophosphate compared to the situation without orthophosphate. This reduction is due to the formation of the insoluble lead orthophosphate compound, which removes lead ions from the solution and decreases their availability for complexation or further reactions.
Overall, the addition of orthophosphate shifts the equilibrium towards the formation of lead orthophosphate, reducing the concentration of free Pb2+ ions in the solution.
learn more about orthophosphate here
https://brainly.com/question/32883978
#SPJ11
Perfluorocarbons (PFCs), hydrocarbons with all H atoms replaced by F atoms, have very weak cohesive forces. One interesting consequence of this property is that a live mouse can breathe while submerged in O₂ -saturated PFCs(c) Rank the kH values in descending order for O₂ in water, ethanol, C₆F₁₄, and C₆H₁₄. Explain your ranking.
Answers
The order of \(k_{H}\) (Henry's Law constant) for O₂ in the molecule is C₆H₁₄ > C₆H₁₄ > ethanol > water.
What is Henry Law constant ?The gas law that states that if the constant is in terms of solubility or pressure.
It is expressed as
\(S_{\text{gas}} = k_{H} \times P_{\text{gas}}\) ...(i)
where,
\(S_{\text{gas}}\) = Solubility of gas
\(k_{H}\) = Henry's Law constant
\(P_{\text{gas}}\) = Partial Pressure of gas
What is Intermolecular force ?Intermolecular force is also called secondary force is the force of attraction between molecules. It acts between ions and atoms.
Water has very strong intermolecular force due to of hydrogen bonding. O₂ gas is least soluble in water.
What is Hydrogen Bonding ?Hydrogen bond is the attractive forces which binds the hydrogen atom of 1 molecule with electronegative atom of other molecule.
Thus from the above conclusion we can say that The order of \(k_{H}\) (Henry's Law constant) for O₂ in the molecule is C₆H₁₄ > C₆H₁₄ > ethanol > water.
Learn more about the Henry Law here: https://brainly.com/question/23204201
#SPJ4
Anything detected with the five senses is considered an
Answers
Answer:
sight, taste, touch, hearing and smell
Explanation:
Anything detected with the five senses is considered a sensory perception or sensory experience
Our five senses are sight (vision), hearing (audition), taste (gustation), touch (tactile perception), and smell (olfaction). These senses allow us to perceive and interpret information from the external environment.
When stated as "detected with the five senses," which means gather information about the world around us through these sensory experiences.
For example:
1. Sight (Vision): We perceive visual information through our eyes, allowing us to see colors, shapes, and movements.
2. Hearing (Audition): We perceive auditory information through our ears, allowing us to hear sounds and distinguish between different tones and pitches.
3. Taste (Gustation): We perceive taste sensations through our taste buds on the tongue, allowing us to distinguish between sweet, sour, salty, bitter, and umami flavors.
4. Touch (Tactile Perception): We perceive tactile sensations through our skin, allowing us to feel textures, pressure, temperature, and pain.
5. Smell (Olfaction): We perceive smells through our olfactory system, located in our nose, allowing us to detect and identify various scents and odors.
All the sensory experiences , whether through sight, hearing, taste, touch, or smell, contribute to our understanding of the world and our surroundings.
Learn more about sensory perceptions here:
https://brainly.com/question/30931333
#SPJ7
A sample of argon has a volume of 5.00 L and the pressure is 0.920 atmIf the final temperature is 303.15 Kthe final volume is 5.70 Land the final pressure is 800mm Hg, what was the initial temperature of the argon?
Answers
Answer:
232.92K
Explanation:
The initial temperature of the argon is 232.9K.Calculation of the initial temperature:Since V₁ = 5dm³P₁ = 0.92atmT₂ = 30°CV₂ = 5.7LP₂ = 800mmHgHere we have to applied the gas lawP1V1/T1 = P2V2/T2Here p, v, and T are pressure, volume and temperature respetivelyAnd, 1 and 2 depict the initial and final states.Now we need to converttemperature to K and pressure into atmSo, it be like T₂ = 30°C = 273 + 30 = 303KP₂ = 800mmHg; 760mmHg = 1 atmSo, = 800/760 = 1.05 atmNow0.92*5 /T = 1.05*5.7/303So, the temperature is 232.92K
Scalar quantities include what 2 things?
A. Number and direction
B. Size and direction
C. Numbers and units
D. Units and directions
Answers
Answer:
D
Explanation:
Units and directions
2. How would type of substance affect the rate of reaction?
Answers
the more the energy, the larger the (a,b,c,d)?
A:crest
B:trough
C:amplitude
D:wavelength
Answers
Answer: D:wavelenght
Explanation: Students will understand that shorter wavelengths have higher frequency and energy.
Answer:
C I believe... I might be wrong though
Explanation:
Arrange the bonds from most Ionic to most covalent in character: Most ionic Most covalent Answer Bank P-Br Br-Br Cl-Br Sr_Br Na-Br
Answers
Ionic and covalent bonds are two types of chemical bonds. An ionic bond is formed between a metal and a nonmetal, while a covalent bond is formed between two nonmetals. Most Ionic: Sr-Br > Na-Br > P-Br > Cl-Br > Br-Br. Most Covalent: Br-Br > Cl-Br > P-Br > Na-Br > Sr-Br
The degree of ionic or covalent character in a bond depends on the electronegativity difference between the atoms that form the bond. The electronegativity difference between the atoms in a bond is a measure of how strongly each atom attracts the shared electrons.
The greater the electronegativity difference, the more ionic the bond will be, and the smaller the electronegativity difference, the more covalent the bond will be.
Using the electronegativity values of the atoms involved, we can rank the bonds from most ionic to most covalent as follows: Most Ionic: Sr-Br > Na-Br > P-Br > Cl-Br > Br-Br. Most Covalent: Br-Br > Cl-Br > P-Br > Na-Br > Sr-Br
Sr-Br and Na-Br are both ionic bonds, with Sr being a more electropositive metal than Na, resulting in a greater electronegativity difference and a more ionic bond. P-Br and Cl-Br are both polar covalent bonds, with Cl being more electronegative than P, resulting in a greater electronegativity difference and a more polar bond.
Finally, Br-Br is a nonpolar covalent bond with no electronegativity difference between the two Br atoms. Overall, the trend in bond character goes from most ionic with the largest electronegativity difference to most covalent with the smallest electronegativity difference.
Know more about electronegativity here:
https://brainly.com/question/17762711
#SPJ11
Ammonium nitrate criss cross formula
Answers
Answer:
By using the criss cross method,the -1 charge of nitrate ion is shifted to ammonium ion and +1 charge of ammonium ion is shifted to nitrate ion. In this way, the final formula NH4NO3 is formed for the ammonium nitrate.
IM IN A TEST PLS HELP
A solution of the acid contains hydrogen ions. Write an ionic equation for the reaction of sodium with the hydrogen ions in the acid. Include state symbols in your answer.
Answers
Answer:
The thing to recognize here is the fact that you're dealing with a neutralization reaction that features sodium hydroxide,
NaOH
, a strong base, and hydrochloric acid,
HCl
, a strong acid.
This tells you that the two reactants will dissociate completely in aqueous solution to produce cations and anions. More specifically, you will have
NaOH
(
a
q
)
→
Na
+
(
a
q
)
+
OH
−
(
a
q
)
HCl
(
a
q
)
→
H
+
(
a
q
)
+
Cl
−
(
a
q
)
Now, when these two solutions are mixed, the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water.
The sodium cations and the chloride anions act as spectator ions because they are present on both sides of the chemical equation as ions.
You can tell that this is the case because sodium chloride,
NaCl
, one of the two products of the reaction, is soluble in aqueous solution.
So, the complete ionic equation will be
H
+
(
a
q
)
+
Cl
−
(
a
q
)
+
Na
+
(
a
q
)
+
OH
−
(
a
q
)
→
Na
+
(
a
q
)
+
Cl
−
(
a
q
)
+
H
2
O
(
l
)
To get the net ionic equation, simply remove the spectator ions
H
+
(
a
q
)
+
Cl
−
(
a
q
)
+
Na
+
(
a
q
)
+
OH
−
(
a
q
)
→
Na
+
(
a
q
)
+
Cl
−
(
a
q
)
+
H
2
O
(
l
)
You will end up with
H
+
(
a
q
)
+
OH
−
(
a
q
)
→
H
2
O
(
l
)
SIDE NOTE You will sometimes see the hydrogen ion being replaced by the hydronium ion,
H
3
O
+
.
In this case, the dissociation of hydrochloric acid is represented by the chemical equation
HCl
(
a
q
)
+
H
2
O
(
l
)
→
H
3
O
+
(
a
q
)
+
Cl
−
(
a
q
)
The net ionic equation will now take the form
H
3
O
+
(
a
q
)
+
OH
−
(
a
q
)
→
2
H
2
O
(
l
)
into which category of hazardous materials do weapons of mass destruction, which utilize chlorine gas, fall?
Answers
Weapons of mass destruction that utilize chlorine gas fall under the category of chemical hazards or chemical weapons.
Weapons of mass destruction (WMD) are highly destructive devices designed to cause significant harm and casualties on a large scale. Chlorine gas, when used as a chemical weapon, falls under the category of chemical hazards.
Chemical hazards refer to substances that can cause harm or pose risks to human health and the environment due to their chemical properties.
Chlorine gas is a toxic and corrosive substance that, when released in large quantities, can have severe detrimental effects on human respiratory systems and other organs. It can cause respiratory distress, lung damage, and even death.
The use of chlorine gas as a weapon is prohibited under international agreements, such as the Chemical Weapons Convention, due to its destructive and inhumane nature.
It is important to note that weapons of mass destruction encompass various types, including chemical, biological, radiological, and nuclear weapons, each with its specific hazards and risks.
Learn more about Chlorine gas here:
https://brainly.com/question/18094198
#SPJ11
Which statement below accurately describes the atoms of a specific element?An antimony, Sb, atom contains 122 protons inside the nucleus and 51 neutrons outside the nucleus.A manganese, Mn, atom contains 55 electrons outside the nucleus and 25 neutrons inside the nucleus.A chlorine, Cl, atom contains 35 electrons and 27 protons inside the nucleus.An arsenic, As, atom contains 33 protons inside the nucleus and 33 electrons outside the nucleus.
Answers
Answer: An arsenic, As, atom contains 33 protons inside the nucleus and 33 electrons outside the nucleus.
Explanation:
The protons are positively charged, electrons are negatively charged and neutrons has no charge (neutral). The protons and neutrons are present inside the nucleus and the electrons are located outside the nucleus.
Antimony (Sb) has an atomic number of 51 and thus contains 51 electrons and 51 protons. It has a mass number of 121 and thus conatins 70 neutrons.
Manganese (Mn) has an atomic number of 25 and thus contains 25 electrons and 25 protons.
Chlorine (Cl) has an atomic number of 17 and thus contains 17 electrons and 17 protons.
Arsenic (As) has an atomic number of 33 and thus contains 33 electrons and 33 protons.
The density of something with these measurements— 2.4 g and 5.12 mL
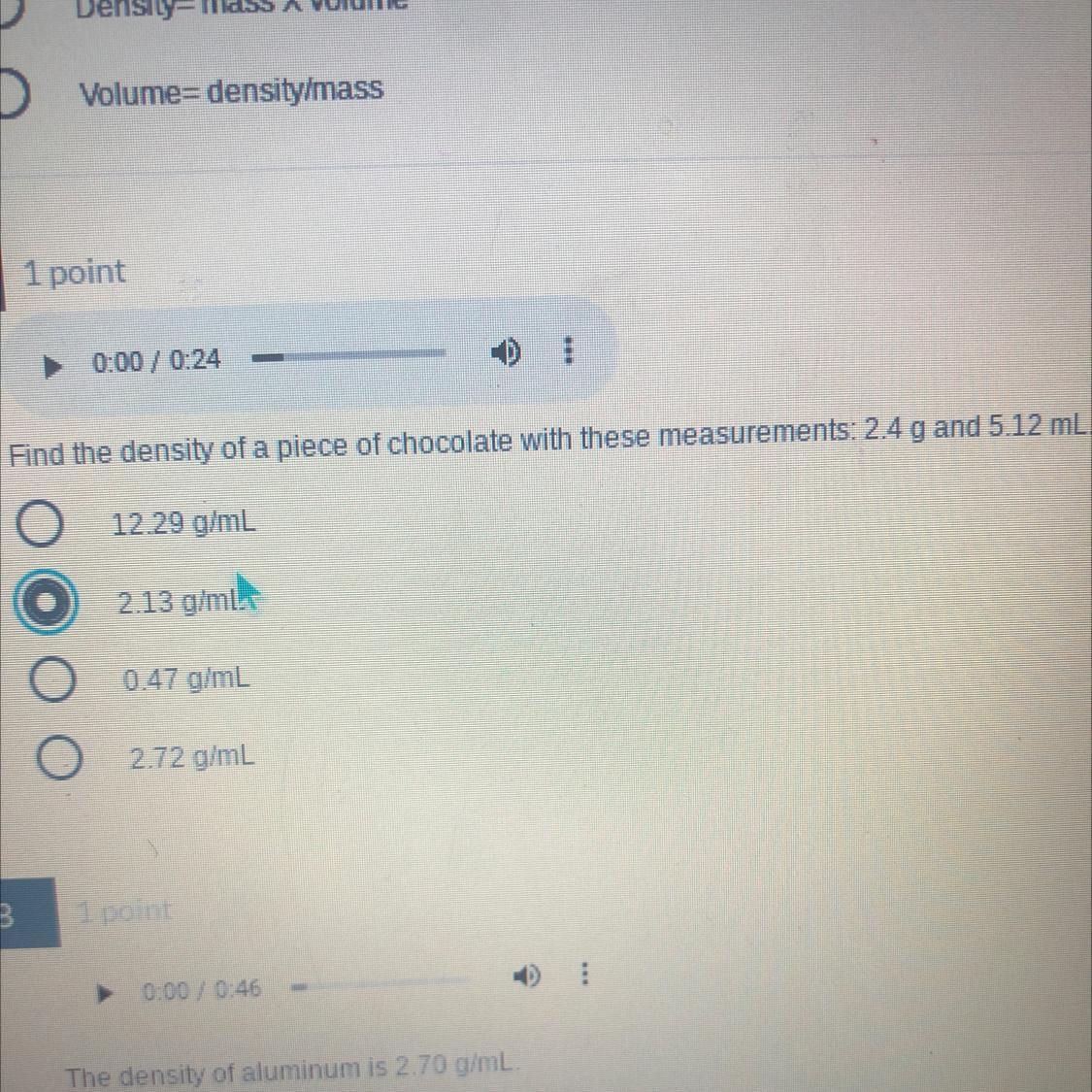
Answers
To find the density of the chocolate, divide the mass by the volume:
\(d=\frac{2.4g}{5.12ml}=\frac{0.47g}{ml}\)The answer is 0.47g/ml.
Which two changes would make this reaction reactant-favored?
2H₂+02 2H₂O + energy
A. Reducing the pressure
B. Increasing the pressure
C. Reducing the temperature
D. Increasing the temperature
Answers
C. Reducing the temperature, is the two changes would make this reaction reactant-favored 2H₂+O₂ 2H₂O + energy
To make the given reaction reactant-favored, we need to shift the equilibrium towards the left side, favoring the formation of reactants (H₂ and O₂) rather than products (H₂O). This can be achieved by considering the impact of pressure and temperature on the reaction.
A. Reducing the pressure:
Reducing the pressure would not favor the reactants. According to Le Chatelier's principle, decreasing the pressure will shift the equilibrium towards the side with a higher number of moles of gas. In this case, both sides of the reaction have the same number of moles of gas (two moles), so reducing the pressure will not have a significant effect.
B. Increasing the pressure:
Increasing the pressure would not favor the reactants either. Again, according to Le Chatelier's principle, increasing the pressure will shift the equilibrium towards the side with fewer moles of gas. As both sides have the same number of moles of gas, changing the pressure will not impact the equilibrium.
C. Reducing the temperature:
Reducing the temperature would favor the reactants. The reaction is exothermic (releases energy), and according to Le Chatelier's principle, decreasing the temperature favors the reaction that produces heat. Therefore, reducing the temperature would shift the equilibrium towards the reactants (H₂ and O₂) side.
D. Increasing the temperature:
Increasing the temperature would not favor the reactants. In an exothermic reaction, increasing the temperature would shift the equilibrium towards the products (H₂O) side to absorb the additional heat.
In conclusion, reducing the temperature (option C) would make the reaction reactant-favored, favoring the formation of H₂ and O₂ rather than H₂O. Therefore, Option C is correct.
Know more about equilibrium here:
https://brainly.com/question/517289
#SPJ8
How much does the temperature of a 28 g sample of water increase by after absorbing
1,174 joules of energy? The specific heat of water is 4.18 J/g x degrees Celsius.
Answers
The temperature of the water sample increases by 8.7 °C after absorbing 1,174 joules of energy.
To calculate the temperature increase of a 28 g sample of water after absorbing 1,174 joules of energy, we need to use the specific heat formula:
\(\[ q = m \cdot c \cdot \Delta T \]\)
Where:
-\(\( q \)\) is the amount of heat energy absorbed (1,174 J in this case)
- m is the mass of the water sample (28 g)
- c is the specific heat of water (4.18 J/g°C)
- \(\( \Delta T \)\) is the change in temperature we want to find
Rearranging the formula to solve for\(\( \Delta T \)\):
\(\[ \Delta T = \frac{q}{m \cdot c} \]\)
Plugging in the given values:
\(\[ \Delta T = \frac{1,174 \, \text{J}}{28 \, \text{g} \times 4.18 \, \text{J/g°C}} = 8.7 \, \text{°C} \]\)
Learn more about temperature here:
brainly.com/question/7510619
#SPJ11
long-chain molecules that consist of many repeating units are called
Answers
The long-chain molecules that consist of many repeating units are called polymers. Polymers are large macromolecules composed of monomer units that are chemically bonded together in a repetitive pattern.
The repeating units, also known as monomers, are linked through covalent bonds, creating a chain-like structure.
Polymers can have various sizes and complexity, ranging from simple structures like polyethylene to highly intricate and specialized macromolecules like proteins and DNA.
It is noticed that due to their repeating nature, polymers often possess unique physical and chemical properties, making them useful in a wide range of applications, including plastics, textiles, adhesives, and biomedical materials.
Read more about Polymers.
https://brainly.com/question/1443134
#SPJ11