Water particles in gas coming off of a pan of boiling water are moving slower than the particles of the water in the pan.
Answers
We want to see what happens to the particle's speed in a given material as the temperature changes and it faces a change of phase.
We will see that the particles in the gas (vapor of water) have a larger speed than the ones in the liquid water.
As we increase the temperature of the material, the kinetic energy of the particles that make the material increase (thus the speed increases). This causes the particles to move more, and this is why as we increase the temperature, is likely to see an increase in the volume.
Then as we increase the temperature of liquid water and that water starts to change of phase (by boiling) the kinetic energy of the particles increase, and thus, the speed increases. then the particles will be moving faster than the particles of the water in the pan.
To know more about kinetic energy visit : https://brainly.com/question/12669551
#SPJ4
Related Questions
Predict the size of a sodium atom
Answers
Answer:
227 pm
Explanation:
Phosphorous acid, H2PHO3, is a diprotic acid. Write equations for the acid ionizations. Write the expressions for Ka1 and Ka2.
Answers
The ionizations of H2PHO3 produce HPO32- and PO43- ions, with respective ionization constants Ka1 and Ka2, in aqueous solutions.
Phosphorous acid, H2PHO3, is a diprotic acid which means it can donate two hydrogen ions (H+) in aqueous solutions. The ionizations of H2PHO3 can be represented as follows:
1. H2PHO3 + H2O ⇌ H3O+ + HPO32-
2. HPO32- + H2O ⇌ H3O+ + PO43-
The first ionization reaction produces the HPO32- ion which is a weak acid that can undergo a second ionization to produce PO43- ion which is a very weak base. The expressions for the ionization constants (Ka) for the two reactions are:
Ka1 = [H3O+][HPO32-]/[H2PHO3]
Ka2 = [H3O+][PO43-]/[HPO32-]
where [H3O+] represents the concentration of hydronium ions, [H2PHO3] represents the concentration of phosphorous acid, [HPO32-] represents the concentration of hydrogen phosphite ions and [PO43-] represents the concentration of phosphate ions.
Learn more about diprotic : https://brainly.com/question/13265808
#SPJ11
Luis and spencer are camping out. each boy decides to build his own fire. spencer brought an ax to chop up a tree limb into foot-long sections for his fuel. luis does not have an ax, so he gathers small branches and breaks them into foot-long pieces. both sources of fuel have an equal mass, and luis and spencer light their fires at the same time. whose fire will last the longest? compare the rate at which each fuel source will burn, and be sure to explain your reasoning. hint: consider the surface area of each fuel source.
Answers
Luis wood will last the longest compared to spencer as his is dry wood it will burn faster and last longer whereas spencer's wood is fresh and has to dry in order to last longer.
A small piece of wood burns more quickly because when something burns, it combines with oxygen to cause combustion. Smaller pieces of wood burn more quickly than larger ones because they provide more surface area for the flame to spread across.
Smaller-diameter pieces should be divided because doing so increases the amount of wood that is exposed. More surface area means faster drying and better burning of the wood. Smaller (3-inch) pieces should be used whole.
to know more about combustion visit
https://brainly.com/question/15117038
#SPJ4
Ionic compounds have a net charge of what?
Answers
Ionic compounds have a net charge of what ?
zero
Any ionic compound will have a net charge of zero. Another way of saying this is that cations and anions must always combine in such a way so that their charges cancel.
Answer:zero
Any ionic compound will have a net charge of zero. Another way of saying this is that cations and anions must always combine in such a way so that their charges cancel.
Explanation:
The first part of the strontium test removes any residual barium. Do you have to be careful adding too much additional chromate? What might happen to the strontium ?
Answers
Yes, it is necessary to be careful when adding too much additional chromate during the strontium test. Excessive amounts of chromate can form a precipitate with strontium ions, leading to the formation of strontium chromate.
This can interfere with the accurate detection and measurement of strontium. Strontium chromate is a yellow solid that can precipitate out of the solution, making it difficult to distinguish and quantify the presence of strontium. This interferes with the accuracy and reliability of the strontium test. Therefore, it is important to use the appropriate amount of chromate in the test to ensure that the reaction specifically targets the barium ions without affecting the strontium ions.
Learn more about chromate here: brainly.com/question/28300460
#SPJ11
My car has an internal volume of 12,000 L. If I drive my car into the river and it implodes, what will be the volume of the gas when the pressure goes from 1.0 atm to 1.4 atm?
Answers
The volume of gas when the pressure goes from 1.0 atm to 1.4 atm is 8,571.43 L.
When a car is driven into the river, it will implode due to the change in pressure. We are to calculate the volume of gas when the pressure goes from 1.0 atm to 1.4 atm if the internal volume of the car is 12,000 L.In order to solve the problem, we will use the combined gas law equation. The equation is given as follows;P1V1/T1 = P2V2/T2where P1 is the initial pressure, V1 is the initial volume, T1 is the initial temperature, P2 is the final pressure, V2 is the final volume, and T2 is the final temperature.We will assume that the initial temperature and final temperature are constant, and therefore, we can cancel them from the equation. Thus, the equation becomes;P1V1 = P2V2We can rearrange the equation to solve for V2 as follows;V2 = (P1V1)/P2Substituting the given values, we get;V2 = (1.0 atm * 12,000 L)/1.4 atmV2 = 8,571.43 L.
For more questions on pressure
https://brainly.com/question/24719118
#SPJ8
How many of the following are buffered solutions? Explain.

Answers
A weak acid as well as its corresponding base make up a buffered solution. The ions are represented by violet, grey, and green spheres, respectively. The first system represents the buffer.
What is buffer?A buffer is indeed a solution that resists pH fluctuations and contains either one weak acid as well as its salt or even a weak base as well as its salt. To put it another way, a buffer is indeed an aqueous solution that includes a weak base as well as its conjugate acid, or a weak acid as well as its conjugate base.
A buffer may also be referred to as a buffer solution, hydrogen ion buffer, and pH buffer. A weak acid as well as its corresponding base make up a buffered solution. The ions are represented by violet, grey, and green spheres, respectively. The first system represents the buffer.
Therefore, the first system represents the buffer.
To know more about buffer, here:
https://brainly.com/question/29763040
#SPJ1
Select the correct answer below that correctly describes what a Arrhenius Acid Base reaction is.
Question 1 options:
An acid base reaction where one compound increases the amount of H+ or OH- in solution.
An acid base reaction where one compound is donating an H+ and one atom is accepting an H+
An acid base reaction where one compound is donating electrons and another compound is accepting electrons.
An acid base reaction where one compound is donating an OH- and one atom is accepting an OH-.
Answers
From the options, the statement ththat describes an acid - base reaction in the Arrhenius sense is; "an acid base reaction where one compound is donating an H+ and one atom is accepting an H+."
What is the Arrhenius definition?The Arrhenius definition of a base is that a base is a substance that releases hydroxide ions as its only negative ions in solution. An acid is a substance that produces hydrogen ions as its only positive ions in solution.
If we look at the definitions shown here, the only one that accurately depicts acid - base reaction in the Arrhenius sense is "an acid base reaction where one compound is donating an H+ and one atom is accepting an H+."
Learn more about Arrhenius definition: https://brainly.com/question/7217496
why must you equalize the pressure inside and outside
Answers
Answer:
You must equalize the pressure inside and outside the flask to determine the total because it keeps the water level the same.
(credits to "coursehero")
Explanation:
Answer: now i don't know
Explanation:
Niels Bohr adapted the nuclear model.
Describe the change that Bohr made to the nuclear model.
Answers
Answer:
To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy.
The diagram shows the electron configuration of an atom of an element for the electrons in the s and p orbitals.
What is the group number of the element in the periodic table?
A) 1
B) 2
C) 13
D) 15
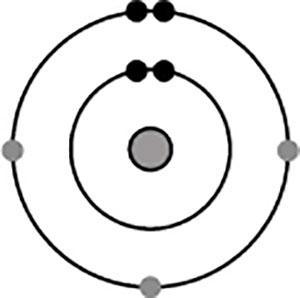
Answers
Answer:
The answer would be 15, letter D.
Explanation:
Since it has 5 electrons in its outermost shell, it will be located in group 15 in the periodic table.
The electron configuration of an atom of an element for the electrons in the s and p orbitals 15th group number of the element in the periodic table option D is correct.
What is electronic configuration of 15th group?Tha common electronic configuration of 15th group elements in ns2 np3, Group 15 elements in the modern periodic table are known as the pnictogens which means suffocation in Greek language.
This group consists of nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi).
The group 15 elements consist of five valence electrons. Due to this the elements can either lose five electrons or gain three electrons in order to attain the stable configuration. The general electronic configuration of nitrogen family is ns 2 np 3.
therefore , because of ns2 np3 option D is correct.
Learn more about electronic configuration , here :
https://brainly.com/question/13497372
#SPJ2
The area of an object is calculated from experimental data to be 24.6623 cm2. The ± absolute error in the area was determined to be ± 0.6 cm2. The area should be reported, in cm 2 , as A. 25 B. 24.7 C. 24.66 D. 24.6623 E. 24.662
Answers
we should take out from point
Mikala pours a tablespoon of salt into a beaker of water. Diffusion will cause the salt molecules to dissolve in the molecules of water. How could Mikala increase the diffusion rate of the salt molecules?
A. Mikala could remove all sources of light from the beaker.
B. Mikala could slowly pour more water into the beaker.
C. Mikala could decrease the temperature of the solution.
D. Mikala could increase the temperature of the solution.
Answers
Explanation- diffusion rates increase at higher temperatures!
Do the following elements represent the same
group, period, or neither?
Li, C, F
F, S, P
O, S, Se
[Choose ]
[Choose ]
[Choose ]
Answers
According to the electronic configuration, Li,C, F belong to same period as they have 2 shells .Among F,S, P it is neither same period or group and for O,S,Se they belong to the same group as they have 6 valence electrons.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ1
What is required for two atoms to share electrons equally in a chemical bend?

Answers
Answer: Nonpolar Covalent Bond
Explanation: A Nonpolar Covalent Bond is created when atoms share their electrons equally. This usually occurs when two atoms have similar or the same electron affinity. The closer the values of their electron affinity, the stronger the attraction. This occurs in gas molecules; also known as diatomic elements.
Describe 3 ways students dispose of garbage at your school
Answers
Answer:
Make Recycle Bins Easily Accessible
Ditch Single-Use Waste
Minimise Paper Waste
Designate a Drawer for Scrap Paper
Eco-Friendly Lunches
What do you call the repetitive motion of a wave (such as the way a cork may bob up and down in the ocean)?
Persistent
Periodic
Propogative
Profundative
Answers
The repetitive motion of a wave will be periodic.
What is repetitive motion?When the action gets repeated, repetitive motion injuries arise. Bending, twisting, gripping, as well as reaching are some examples.
What is periodic?A periodic expression is one whose values repeat at regular intervals.
What is wave?A pulse is just a single disturbance, while a wave would be a continuous and repeated interruption of a medium.
The wave can be described as the disturbance water's surface that moves up and down. As even the wave crests, it forces a seagull to fly vertically and horizontally in a simple harmonic motion.
Therefore, the repetitive motion of a wave will be periodic.
To know more about wave , repetitive motion and periodic
https://brainly.com/question/9624826
#SPJ2
How many seconds are in 2.83 days? do not type any units, just the correct answer with the correct sig figs. use commas to separate every 3 places. for example: 128,000 s not 128000 s (2 points)_
Answers
There are 244,512 sec in 2.83 days.
what are significant figures? What are rules of it?Significant figures are the number of digits in a value , often a measurement,that contribute to the degree of accuracy of the value.
Rules of significant figures are :
1) All non zero digits are significant.
2) Zeros between non zero are significant.
3) leading zeros are never significant.
4) In a number with a decimal point, trailing Zeros,those to the right of the last non zero digit ,are significant.
We have 24 hrs in a day and 1 hr contains 3600 sec so,
→2.83×24×3600
→244,512 seconds in 2.83 days.
to learn more about Significant figures click here https://brainly.com/question/14359464
#SPJ4
An experiment was designed to test the hypothesis that peanuts have more energy than a chip. The experiment determines calorimetry of the peanut and the chip, using water to capture the energy of the samples when they were burned. A 10 g sample of each type of food was burned underneath a metal can that held 50 g of water. A thermometer captured how much the temperature of the water increased. The water increased by 7 degrees for the peanut and 3 degrees for the chip. What conclusion can be drawn?
The chip has more energy than the peanut.
The peanut has more energy than the chip.
The experiment was flawed because more than one variable was being tested.
The peanut and the chip have the same amount of energy.
Answers
Since the water increased by 7 degrees for the peanut and 3 degrees for the chip, the conclusion would be that the peanut has more energy than the chip.
CalorimetryCalorimetry is the science of measuring the quantity of energy, usually in the form of heat, that is released during a reaction.
In this case, the energy released by the peanut and the chip were measured using calorimetry. The energy released was captured by the water and helps raise the temperature of the water.
The more energy released by the substances, the more the temperature of the water is raised.
Thus, since the peanut raised the temperature of the water by 7 degrees while the chip was only able to raise it by just 3 degrees, one can effectively conclude that the peanut has more energy than the chip.
More on calorimetry can be found here: https://brainly.com/question/11477213
#SPJ1
Calculate the mass of solid required to make 750.0ml of0.20 mol/L solution of lead ii nitrate
Answers
The question requires us to calculate the mass necessary to prepare a lead(II) nitrate (Pb(NO3)2) solution, given the volume of the solution and its molar concentration.
The following information was provided by the question:
salt used = Pb(NO3)2
volume of solution = V = 750.0 mL = 0.7500 L
molar concentration of solution = C = 0.20 mol/L
To solve this problem, we'll use two important definitions and their equations: the molar concentration and number of moles.
The molar concentration (C, given in mol/l) is defined as the number of moles of a compound (n, given in moles) divided by the volume of the solution (V, given in liters):
\(C=\frac{n\text{ (mol)}}{V\text{ (L)}}\)On the other hand, the number of moles of a substance (n, given in moles) can be calculated by dividing the mass of this substance (m, given in grams) by its molar mass (M, given in g/mol):
\(n=\frac{m\text{ (g)}}{M\text{ (g/mol)}}\)If we replace n as given by the second equation in the first equation, we'll have:
\(C=\frac{m(g)}{M(g/mol)\times V(L)}\)And, rearranging this last equation, we find an expression to calculate the mass of a substance from the molar concentration of a solution, its volume and the molar mass of the substance:
\(m=C\times M\times V\)(where m is the mass, in g, C is the molar concentration, in mol/L, M is the molar mass, in g/mol, and V is the volume, in L).
Since the question provided the values for C and V, we'll only need the molar mass of Pb(NO3)2.
The atomic masses of Pb, N and O are 207.2 u, 14.01 u and 15.99 u, respectively. Thus, the molar mass of Pb(NO3)2 can be calculated as:
molar mass Pb(NO3)2 = (1 * 207.2) + (2 * 14.01) + (6 * 15.99) = 331.2 g/mol
Next, we apply the values of to the equation to calculate the mass of Pb(NO3)2:
\(m=C\times M\times V\to m=(0.20\text{ mol/L)}\times(331.2\text{ g/mol)}\times(0.7500\text{ L) = }49.68\text{ g}\)Therefore, the mass of Pb(NO3)2 required to prepare 750.0 mL of a 0.20 M solution is 49.68 g.
the burden of proof required in a criminal case is
Answers
The burden of proof required in a criminal case is "beyond a reasonable doubt."
In a criminal case, the burden of proof refers to the legal obligation of the prosecution to prove that the defendant committed the crime they are accused of. The standard of proof required in criminal cases is "beyond a reasonable doubt."This means that the prosecution must present evidence that is strong enough to convince the jury or judge of the defendant's guilt to a level that is beyond any reasonable doubt.
If there is any reasonable doubt in the mind of the jury or judge, then the defendant must be acquitted of the charges against them. The burden of proof in a criminal case is very high because the consequences of a criminal conviction can be very serious, including imprisonment, fines, and other penalties. It is therefore important that the prosecution presents a strong and compelling case that meets the "beyond a reasonable doubt" standard in order to secure a conviction.
Learn more about criminal case here:
https://brainly.com/question/31580381
#SPJ11
calculate the current needed to deposit 2.45 g of ni in 3 hours and 15 minutes from a ni(no3)2 solution
Answers
The current needed to deposit 2.45 g of ni in 3 hours and 15 minutes from a ni(no3)2 solution is approximately 0.084 amperes.
The first step in solving this problem is to determine the amount of charge required to deposit 2.45 g of ni. This can be done using Faraday's law, which states that the amount of charge required to deposit a given amount of a substance is proportional to its atomic weight and the number of electrons involved in the reaction.
The atomic weight of ni is 58.69 g/mol, and each ni ion requires two electrons to be deposited. Therefore, the amount of charge required to deposit 2.45 g of ni can be calculated as follows:
(2.45 g)/(58.69 g/mol) x (2 electrons/ni) x (1 mole/6.022 x 10^23 electrons) = 2.501 x 10^20 electrons
The next step is to determine the time required to deposit this amount of charge. This can be done using the equation:
Q = It
where Q is the amount of charge (in coulombs), I is the current (in amperes), and t is the time (in seconds).
Since we are given the time in hours and minutes, we need to convert it to seconds:
3 hours and 15 minutes = (3 x 60 x 60) + (15 x 60) = 11,700 seconds
Substituting the values we have calculated into the equation, we get:
2.501 x 10^20 electrons = I x 11,700 seconds
Solving for I, we get:
I = 2.501 x 10^20 electrons/11,700 seconds = 0.084 amperes
Therefore, the current needed to deposit 2.45 g of ni in 3 hours and 15 minutes from a ni(no3)2 solution is approximately 0.084 amperes.
To learn more about Current, visit;
https://brainly.com/question/1100341
#SPJ11
Is H2PO3 ionic, covalent or a acid?
Answers
50.) a 26 m tall statue of buddha in tibet is covered with 279 kg of gold. if the gold
was applied to a thickness of 0.0015 mm, what surface area (in square units) was
covered? [gold's density is 19,320 kg/m?]
Answers
The surface area of the statute covered with the gold is 9,627.32 m².
What is Volume?
Volume is a scalar quantity expressing the amount of three-dimensional space enclosed by a closed surface.
Volume of the statute covered with gold
The volume of the statute covered with gold is calculated as follows;
Volume = mass/density
Volume = (279 kg) / (19,320 kg/m³)
Volume = 0.0144 m³
Surface area of the statute covered with goldThe surface area of a solid object is a measure of the total area that the surface of the object occupies.
V = S.A x h
where;
S.A is surface areah is thicknessS.A = V/h
S.A = (0.0144) / (0.0015 x 10⁻³)
S.A = 9,627.32 m²
Thus, the surface area of the statute covered with the gold is 9,627.32 m².
Learn more about surface area here: https://brainly.com/question/76387
#SPJ1
Reactive Nonmetal
A. Cadmium
B. Titanium
C. Oxygen
D. Krypton
Answers
If energy is "transferred out" of a substance, what causes the
molecules to change phase faster?
Answers
alance the following redox reaction in acidic solution: mno^-4−(aq) so2(g)⟶mn^2+ (aq) so4^2−4(aq)
Answers
So, the balanced equation for the redox reaction in acidic solution is:
\(2MnO_4^-(-4)(aq) + SO_2(g) == 2Mn^2+ (aq) + SO_4^2-4(aq)\)
To balance the following redox reaction in acidic solution:
\(2MnO_4^-(-4)(aq) + SO_2(g) == 2Mn^2+ (aq) + SO_4^2-4(aq)\)
We need to add coefficients in front of each reactant and product in the balanced equation to make the number of atoms of each element on both sides of the equation equal.
First, we need to write the balanced equation for the reaction:
\(2MnO_4^-(-4)(aq) + SO_2(g) == 2Mn^2+ (aq) + SO_4^2-4(aq)\)
Next, we need to add coefficients in front of each reactant and product to balance the equation. The coefficients indicate the number of moles of each substance present in the reaction.
Coefficients: In this balanced equation, the number of atoms of each element on both sides of the equation is equal.
Therefore, the balanced equation for the redox reaction in acidic solution is:
\(2MnO_4^-(-4)(aq) + SO_2(g) == 2Mn^2+ (aq) + SO_4^2-4(aq)\)
Learn more about balanced equation visit: brainly.com/question/26694427
#SPJ4
Please help me on this exsam question ASAP!!! Please

Answers
Well insulated houses use less energy to keep them warm. This means that less carbon dioxide, a greenhouse gas, is released into the atmosphere from burning of fossil fuels (coal, oil and gas) in our power stations and central heating boilers. Global warming is occurring because large volumes of greenhouse gases – including carbon dioxide – are accumulating in the Earth’s atmosphere. This causes the planet’s average air and ocean temperatures to increase and sea levels to rise, resulting in increasingly severe weather conditions. This effect is called climate change and it’s already happening. By improving the insulation of your home, you will be playing your part in reducing the effect of global warming.
What is the term for propane and butane fases that can be liquified?
Answers
The term for the propane and butane phases that can be liquefied is "liquefied petroleum gas" or LPG. LPG is a mixture of propane and butane gases that are compressed and cooled to a point where they transition from their gaseous state to a liquid state.
This process of converting the gases into a liquid form allows for easier storage, transportation, and handling. LPG is commonly used as a fuel for heating, cooking, and powering various appliances. It is widely available in portable cylinders and larger storage tanks. LPG has a higher energy content compared to its gaseous form, making it a convenient and efficient fuel source. The ability of propane and butane to be liquefied and stored as LPG is due to their relatively low boiling points and the pressure at which they are compressed. By controlling the temperature and pressure, the gases can be condensed into a liquid state, allowing for greater convenience and versatility in their use.
Learn more about liquefied petroleum gas here: brainly.com/question/20364043
#SPJ11
nitration of methyl benzoate how to create more electrophile ?
Answers
Nitration is the process by which an nitro group (-NO2) is introduced to a chemical compound. Electrophile is a molecule that has a tendency to acquire electrons and hence it is attracted towards the electron-rich centers to neutralize the charge imbalance.
During the nitration of methyl benzoate, the reaction is carried out with nitronium ion (NO2+), which is highly electrophilic and attacks the aromatic ring. The nitration of methyl benzoate occurs in the presence of a mixture of concentrated sulfuric acid and concentrated nitric acid (nitrating mixture).The nitrating mixture is used to prepare the nitronium ion, NO2+. This is the electrophile which carries out the nitration of methyl benzoate.Nitronium ion is formed as follows: HNO3 + H2SO4 → NO2+ + HSO4− + H2OWhen sulfuric acid is added to nitric acid, the acid becomes protonated and undergoes an equilibrium reaction as shown below:HNO3 + H2SO4 ⇌ NO2+ + HSO4− + H2OThe product that is formed is nitronium ion, NO2+. Thus, by adding sulfuric acid, the concentration of NO2+ increases which increases the electrophilicity and leads to the formation of more electrophile. Therefore, the concentration of the nitronium ion can be increased by adding more sulfuric acid to the reaction mixture, which will make the solution more acidic, increasing the amount of nitronium ion, NO2+.
For more information on Nitration visit:
brainly.com/question/29789429
#SPJ11