What are likely formulas for the following molecules?
(a) NH2OH
(b) AlCl2
(c) CF2Cl2
(d) CH2O
Answers
(a) NH2OH- Nitrogen is a group 5 element and has 5 valence electrons. Oxygen is a group 6 element and has 6 valence electrons. Hydrogen is a group 1 element and has 1 valence electron. Therefore, NH2OH has the formula: NH2OH
(b) AlCl2- Aluminum is a group 3 element and has 3 valence electrons. Chlorine is a group 7 element and has 7 valence electrons. Therefore, AlCl2 has the formula: AlCl2
(c) CF2Cl2- Carbon is a group 4 element and has 4 valence electrons. Fluorine is a group 7 element and has 7 valence electrons. Chlorine is a group 7 element and has 7 valence electrons. Therefore, CF2Cl2 has the formula: CF2Cl2
(d) CH2O- Carbon is a group 4 element and has 4 valence electrons. Oxygen is a group 6 element and has 6 valence electrons. Hydrogen is a group 1 element and has 1 valence electron. Therefore, CH2O has the formula: CH2O
To know more about Nitrogen refer to-
brainly.com/question/16711904#
#SPJ11
Related Questions
a catalyst is a substance which speeds up a chemical reaction and is also consumed during the reaction.
Answers
A catalyst is a substance that speeds up a chemical reaction and is also consumed during the reaction.
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with lower activation energy. It accomplishes this by facilitating the formation of intermediate species and reducing the energy barrier for the reaction to occur. The catalyst itself undergoes a reaction with the reactants, forming an intermediate complex, but it is regenerated and recovered in its original form at the end of the reaction.
The role of a catalyst can be explained through a simplified reaction mechanism. Consider a hypothetical reaction between reactants A and B to form product C. Without a catalyst, the reaction may proceed slowly due to a high activation energy. However, when a catalyst is introduced, it provides an alternative pathway with a lower activation energy.
The catalyst initially binds to the reactants, forming a catalyst-reactant complex. This complex undergoes rearrangements and reactions that lead to the formation of the product. At the end of the reaction, the catalyst is regenerated, allowing it to participate in subsequent reaction cycles.
The consumption of the catalyst occurs during the reaction, as it reacts with the reactants to form intermediates and ultimately the products. However, the catalyst is not permanently altered or consumed entirely. Instead, it is regenerated and can be reused in subsequent reactions.
In summary, a catalyst is a substance that accelerates a chemical reaction by providing an alternative reaction pathway with lower activation energy. It is consumed during the reaction as it reacts with the reactants to form intermediates and products. However, the catalyst is not permanently consumed and can be regenerated for reuse in subsequent reactions.
To know more about catalyst, visit:
https://brainly.com/question/666246
#SPJ11
Practice Run Just need a bit of help! Good amount of points!
According to LeChatelier's principle, what are 3 general ways that you can reverse a reaction when it is at equilibrium. Select all that apply.
Adding concentration of product
Adding concentration of product
Adding concentration of reactant
Adding concentration of reactant
Keeping the concentration the same
Keeping the concentration the same
Changing temperature
Changing temperature
Changing the state of matter
Changing the state of matter
Changing the color
Changing the color
Changing Pressure
Answers
The 3 general ways by which a system in equilibrium can be reversed are by changing the concentration of the reactants of products, changing the pressure of the system, and changing the temperature of the system.
Le Chatelier's principleLe Chatelier's principle state that when a reaction is in equilibrium and one of the constraints that affect the rate of reactions is applied to the system, the equilibrium shifts so as to cancel out the effects of the constraints.
The constraints being referred to by Le Chatelier are concentration, pressure, and temperature. Increasing or decreasing the pressure of a system in equilibrium will shift the equilibrium to the sides with the lower moles or higher moles respectively.
Increasing the concentration of the reactants will shift the equilibrium toward the product side while increasing the concentration of the products will shift the equilibrium toward the reactant side.
In the same vein, increasing the temperature of a system will sift the equilibrium towards the product if the system itself is endothermic. If the system is exothermic, a reversal will occur.
More on Le Chatelier's principle can be found here: https://brainly.com/question/2001993
#SPJ1
Figure A and Figure B represent examples of different types of chemical bonding. Identify the descriptions and properties that best represent each figure. All of the descriptions and properties may not be used. Figure A Figure B ________ ________
Answer Bank - Na-Clbond - nonpelar covale ionic - CI-CI bond - transder of cloctrom - N-H bond - polar covalent - cual sharing of clectrons - unoqual sharing of electrons
Answers
As for the two different types of chemical bonding illustrated by Figure A and Figure B, they are the Ionic bond and Polar covalent bond. The properties and descriptions that best illustrate each figure are listed below:Figure AIonic BondThe ionic bond involves the transfer of valence electrons from the nonmetal to the metal ion. There is no sharing of electrons in ionic bonding, and the valence electrons in the anion are transferred to the metal cation, creating an ion-pair. This leads to an electrostatic attraction between the anion and cation, which is the ionic bond's fundamental concept.The Na-Cl bond is a prime example of an ionic bond since sodium is a metal, while chlorine is a nonmetal, and the bond between them is ionic.Polar Covalent BondThe polar covalent bond involves unequal sharing of electrons, as opposed to the ionic bond. Electronegativity differences lead to unequal sharing, and if one atom is more electronegative than the other, it attracts electrons closer to it, creating partial negative and positive charges.The N-H bond is a perfect example of a polar covalent bond because the hydrogen atom has a partial positive charge, while the nitrogen atom has a partial negative charge.Figure BNon-polar Covalent BondA non-polar covalent bond occurs when two atoms share electrons equally between them. Both atoms have the same electronegativity; thus, electrons are evenly shared between them.The C-Cl bond is a prime example of a non-polar covalent bond since both atoms have a relatively similar electronegativity, and there is an equal sharing of electrons.Transder of CloctromThere is no such thing as a transder of cloctrom bond. The correct term is transfer of electrons.CI-CI BondCI-CI bond is a covalent bond between two identical chlorine atoms, representing a diatomic molecule. Since the atoms are identical, there is no difference in electronegativity, and electrons are evenly distributed.Polar Covalent BondThe polar covalent bond involves unequal sharing of electrons, as opposed to the ionic bond. Electronegativity differences lead to unequal sharing, and if one atom is more electronegative than the other, it attracts electrons closer to it, creating partial negative and positive charges.The unequal sharing of electrons is best illustrated by the unequal sharing of electrons in the carbon-oxygen bond.
We have air (21% O2 and 79% N2) at 23 bar and 30 C. 4. What is the ideal molar volume (m^3/kmol)? a. b. What is the Z factor? What is the real molar volume?
Answers
Answer:
The ideal molar volume is \(\frac{V}{n} =V_z= 0.001095 \ m^3/mol\)
The Z factor is \(Z = 0.09997\)
The real molar volume is \(\frac{V_r}{n} = V_k= 0.0001095\ \frac{m^3}{mol}\)
Explanation:
From the question we are told that
The pressure is \(P = 23 \ bar = 23 *10^5 Pa\)
The temperature is \(T = 30 ^ oC = 303 \ K\)
According to the ideal gas equation we have that
\(PV = nRT\)
=> \(\frac{V}{n}=V_z= \frac{RT}{P}\)
Where \(\frac{V}{n }\) is the molar volume and R is the gas constant with value
\(R = 8.314 \ m^3 \cdot Pa \cdot K^{-1}\cdot mol^{-1}\)
substituting values
\(\frac{V}{n} =V_z= \frac{ 8.314 * 303}{23 *10^{5}}\)
\(\frac{V}{n} =V_z= 0.001095 \ m^3/mol\)
The compressibility factor of the gas is mathematically represented as
\(Z = \frac{P * V_z}{RT}\)
substituting values
\(Z = \frac{23 *10^{5} * 0.001095}{8.314 * 303}\)
\(Z = 0.09997\)
Now the real molar volume is evaluated as
\(\frac{V_r}{n} = V_k= \frac{Z * RT }{P}\)
substituting values
\(\frac{V_r}{n} = V_k= \frac{0.09997 * 8.314 * 303}{23 *10^{5}}\)
\(\frac{V_r}{n} = V_k= 0.0001095\ \frac{m^3}{mol}\)
write the number 874.591 correct to 2 decimal places
Answers
Answer:
874.59
Explanation:
The number after 9 is 1
1 is lower than 5 so we leave the previous number the same
The number 874.591 correct to 2 decimal places is 874.59.
What is a decimal number?A decimal is a fraction written in a special shape. as opposed to writing 1/2, for example, you could express the fraction because the decimal 0.5, where the 0 is inside the one vicinity and the 5 is within the tenths vicinity. Decimal comes from the Latin phrase decimus, meaning tenth, from the root phrase decem, or 10.
What are the types of decimals?terminating Decimal Numbers, non-terminating Decimal Numbers, recurring Decimal Numbers, non-recurring Decimal Numbers.Learn more about decimals here: https://brainly.com/question/703656
#SPJ2
Use the following information to answer numbers 20-22: Start with a 30M sucrose solution. Then you do a serial dilution by making 100 mL of a 1/10 dilution and repeat THREE more times. 20. Show the calculation for the first dilution. (1 pt) Answer: 21. Draw and label a diagram of the serial dilution with volumes and concentrations of the stock and dilution beakers. (2 pts) Answer: 22. Show your concentration calculations. (1 pt) Answer:
Answers
The concentrations for the second, third, and fourth dilutions would be 0.3M, 0.03M, and 0.003M, respectively.
In a serial dilution, a concentrated solution is successively diluted to obtain solutions with lower concentrations. In this case, starting with a 30M sucrose solution, a series of four 1/10 dilutions are performed. The first dilution involves making 100 mL of the 1/10 dilution. To answer question 20, the calculation for the first dilution needs to be shown. For question 21, a diagram of the serial dilution with labeled volumes and concentrations of the stock and dilution beakers needs to be drawn. Finally, for question 22, the concentration calculations for each dilution step need to be provided.
To calculate the first dilution, we need to determine the concentration of the resulting solution. Since it is a 1/10 dilution, the concentration would be 1/10 times the original concentration. Therefore, the concentration of the first dilution would be 30M / 10 = 3M.
For question 21, a diagram needs to be drawn to illustrate the serial dilution process. The diagram should include the stock solution with a volume of 30M, the first dilution beaker with a volume of 100 mL and a concentration of 3M, and labels indicating the volumes and concentrations at each step of the dilution process.
For question 22, the concentration calculations for each dilution step need to be provided. Starting with the first dilution at 3M, subsequent dilutions would be 1/10 of the previous concentration. Therefore, the concentrations for the second, third, and fourth dilutions would be 0.3M, 0.03M, and 0.003M, respectively.
Overall, these calculations and the diagram represent the process and concentrations involved in the serial dilution of the 30M sucrose solution.
To learn more about serial dilution click here: brainly.com/question/28548168
#SPJ11
What problems might it cause if a company tried to recycle materialswithout sorting them first?
Answers
This is the last one I need. Just want to make sure I did it right.

Answers
To combine ions to form ionic compounds, we need the combine in such a way that it gets neutral charge.
We can combine each anion with each cation to get the 4 compounds we need.
To combine SO₄²⁻ with Pb⁴⁺ we first find the Least Common Multiple of their charges, 2 and 4.
They have the factor 2 in common, so the LCM is 4. This is the final charge of each that will cancel out.
To get 4+, we only need 1 Pb⁴⁺.
To get 4-, we need 2 SO₄²⁻.
So, the formula is:
Pb(SO₄)₂
To combine SO₄²⁻ with NH₄⁺ is easier because one of them has single charge. In this case, we can simply pick one of the multiple charge ion and the same amount that will cancel its charge of the single charged one.
So, we picke 1 SO₄²⁻, ending with 2-.
And we picke 2 NH₄⁺, ending with 2+.
The formula:
(NH₄)₂SO₄
To combine C₂H₃O₂⁻ with Pb⁴⁺ we do the same, because the anion is single charged.
Pick 1 Pb⁴⁺, ending with 4+.
Pick 4 C₂H₃O₂⁻, ending with 4-.
The formula:
Pb(C₂H₃O₂)₄
To combine C₂H₃O₂⁻ with NH₄⁺, both have same charge, so we just need one of each and their charges will cancel out.
The formula:
NH₄C₂H₃O₂
So, the formulas are:
Pb(SO₄)₂
(NH₄)₂SO₄
Pb(C₂H₃O₂)₄
NH₄C₂H₃O₂
how many atoms are in hydrogen
Answers
Answer:6.02
Explanation:
Answer:
there are about 6 atoms in hydrogen
Ammonium phosphate ((NH4), PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H;PO,) with ammonia (NH3)
What mass of ammonium phosphate is produced by the reaction of 8.29 g of phosphoric acid?
Round your answer to 3 significant digits.
Answers
Explanation:
We have to first write an equation for the reaction that occurs:
H₃PO₄ + 3 NH₃ → (NH₄)₃PO₄
Now, moles of H₃PO₄ = mass ÷ molar mass
= 8.29 g ÷ 97.99 g/mol
= 0.0846 mol
Now, the mole ratio of H₃PO₄ : (NH₄)₃PO₄ is 1 : 1
∴ since moles of H₃PO₄ = 0.0846 mol
then moles of (NH₄)₃PO₄ = 0.0846 mol
∴ the mass of (NH₄)₃PO₄ produced = mol × molar mass
= 0.0846 mol × 149.09 g/mol
= 12.6 g
What is the amount of aluminum chloride produced from 40 moles of chlorine and excess aluminum?
Answers
Answer: Molar mass of Al = 26.98 g/mol
mass of Al = 12 g
mol of Al = (mass)/(molar mass)
= 12/26.98
= 0.4448 mol
According to balanced equation
mol of AlCl3 formed = moles of Al
= 0.4448 mol
Answer: 0.445 mol
hope this help boo❤️❤️❤️
Explanation:
Animals need various nutrients in order to live and grow. Nitrogen, for example, is a nutrient that is needed to form molecules found in genetic material. How do animals obtain nitrogen? A. They eat plants which have absorbed nitrogen from the soil. B. They create nitrogen in their bodies using other nutrients as building blocks. C. They breathe in and absorb nitrogen gas from the atmosphere. D. all of these
Answers
Answer:
It's A
Explanation:
Animals get the nitrogen they need by eating plants or other animals that contain nitrogen. When organisms die, their bodies decompose bringing the nitrogen into soil on land or into ocean water. Bacteria alter the nitrogen into a form that plants are able to use.
When copper (II) chloride reacts with sodium nitrate, NaNO3, copper (II) nitrate, Cu(NO3)2, and sodium chloride are formed. Write a balanced chemical equation for this reaction. Then determine how much sodium chloride can be formed when 15.0 grams of copper (II) chloride react with 20.0 grams of sodium nitrate.
Answers
Answer:
CuCl2 + 2NaNO3 → Cu(NO3)2 + 2NaCl
Explanation:
m(NaCl) =m(CuCI2) × 2Mr(NaCl) / Mr(CuCI2)
= 15 × 2 × (94/135)
=20.89 g
which surface has most friction

Answers
Explain why potassium is more reactive than lithium
Answers
What does the little ‘2’ in 2O2 mean?
Answers
Answer:200
Explanation:
What is the chemical name of NaCl₂
Answers
Answer:
It’s sodium chloride
Explanation:
Hope that helps
convert 256 ml to kl
Answers
Answer:
0.000256
Explanation:
Formula:
Divide the value volume by 1e+6
1 kl equal to 1000000ml
what's the answerrr?? :)
Magnesium ribbon reacts with dilute hydrochloric acid. Explain how altering the concentration of the hydrochloric acid alters the rate of the reaction???? (3 marks)
Answers
Explanation:
The reaction between magnesium ribbon and dilute hydrochloric acid is a classic example of a single replacement reaction, which can be represented by the following chemical equation:
Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
In this reaction, magnesium (Mg) reacts with hydrochloric acid (HCl) to form magnesium chloride (MgCl2) and hydrogen gas (H2).
The rate of this reaction can be altered by changing the concentration of the hydrochloric acid. This is because the rate of a chemical reaction depends on the concentration of the reactants. Specifically, the rate of the reaction is proportional to the concentration of the reactants raised to some power, which is determined by the reaction's rate law.
In this reaction, the rate law can be expressed as:
Rate = k [Mg] [HCl]^x
Where k is the rate constant and x is the order of the reaction with respect to hydrochloric acid. The order of the reaction with respect to magnesium is one, since the concentration of magnesium does not change during the reaction.
When the concentration of hydrochloric acid is increased, the rate of the reaction increases because there are more hydrochloric acid molecules available to collide with magnesium atoms and react. This means that the value of x is greater than zero and the reaction is dependent on the concentration of hydrochloric acid.
Conversely, when the concentration of hydrochloric acid is decreased, the rate of the reaction decreases because there are fewer hydrochloric acid molecules available to react with magnesium. This means that the value of x is less than one and the reaction is not entirely dependent on the concentration of hydrochloric acid.
5. find the concentration of 100.0 ml of hcl if 80.0 ml of 2.5 m naoh is required to neutralize the acid. a) how many moles of base were added to the beaker to neutralize the acid? b) how many moles of acid were originally in the beaker? c) using the original moles of acid and the original volume of acid in the flask, calculate the molarity of the hcl.
Answers
To find the concentration of HCl, we need to calculate the moles of base added to neutralize the acid, the moles of acid originally in the beaker, and then use these values to determine the molarity of HCl.
a) To find the moles of base (NaOH) added, we can use the formula:
Moles of NaOH = Volume of NaOH (in L) × Molarity of NaOH
Converting the volume to liters and using the given values:
Moles of NaOH = 0.080 L × 2.5 mol/L = 0.2 mol
b) Since the reaction is a 1:1 stoichiometric ratio between NaOH and HCl, the moles of acid (HCl) will be equal to the moles of base added. Therefore, there were also 0.2 mol of HCl originally in the beaker.
c) Now, we can calculate the molarity of HCl using the formula:
Molarity (M) = Moles of solute / Volume of solution (in L)
Given that the volume of the acid is 100.0 mL (or 0.100 L) and the moles of acid is 0.2 mol:
Molarity of HCl = 0.2 mol / 0.100 L = 2.0 M
Therefore, the molarity of the HCl solution is 2.0 M.
Learn more about the molarity here: brainly.com/question/30849828
#SPJ11
qiuzlet explain how sodium bicarbonate, nahco3, functions as an antacid. be sure to include a chemical reaction in your answer.
Answers
The Sodium bicarbonate, also known as nahco3, functions as an antacid by neutralizing excess stomach acid. When there is too much acid in the stomach, it can cause discomfort and heartburn. Sodium bicarbonate reacts with the acid to form carbon dioxide, water, and a salt. The chemical reaction can be represented by the equation. NaHCO3 + HCl → NaCl + CO2 + H2O.
The equation, NaHCO3 sodium bicarbonate reacts with HCl hydrochloric acid to form NaCl sodium chloride, also known as table salt, CO2 carbon dioxide, and H2O water. The carbon dioxide produced helps to relieve the discomfort caused by excess stomach acid. Overall, the reaction helps to balance the pH level in the stomach and reduce acidity. Sodium bicarbonate, NaHCO3, functions as an antacid by neutralizing excess stomach acid. The primary acid in your stomach is hydrochloric acid HCl, which helps to break down food. When there's too much acid, it can cause discomfort and heartburn. Here's the step-by-step explanation of how sodium bicarbonate acts as an antacid, including the chemical reaction Sodium bicarbonate NaHCO3 dissolves in water and dissociates into Na+ and HCO3-. The bicarbonate ion HCO3- reacts with the hydrochloric acid HCl in your stomach. This reaction produces water H2O, carbon dioxide CO2, and sodium chloride NaCl.The chemical reaction can be represented asHCO3- aq + HCl aq → H2O I + CO2 g + NaCl aq This neutralization reaction reduces the acidity in your stomach, providing relief from the symptoms associated with excess stomach acid.
learn more about bicarbonate here
https://brainly.com/question/8560563
#SPJ11
the smoke inhaled from a burning cigarette contains a mix of over how many chemicals?
Answers
The smoke inhaled from a burning cigarette contains a mix of over 7,000 chemicals, including nicotine, tar, and carbon monoxide, many of which are toxic and carcinogenic.
How does smoking affect the body?Smoking can cause a wide range of health problems, including lung cancer, heart disease, stroke, respiratory infections, and chronic obstructive pulmonary disease (COPD). It can also affect the reproductive system, increase the risk of osteoporosis, and damage the skin and teeth.
What are some strategies for quitting smoking?There are several strategies that can be effective in quitting smoking, including nicotine replacement therapy, medication, counseling, and support groups. Quitting smoking can be challenging, but it can significantly improve overall health and reduce the risk of serious health problems.
To know more about smoke,visit:
https://brainly.com/question/1218871
#SPJ1
How many moles of electrons will be removed from methane when a methane oxidizing organism converts 119 moles of methane (ch4) to carbon dioxide gas (co2) during respiration?
Answers
moles of electrons will be removed from methane when a methane oxidizing organism converts 119 moles of methane (CH₄) to carbon dioxide gas (CO₂) during respiration would be 119 as well. Each mole of methane (CH4) that is converted to carbon dioxide (CO2) during respiration yields
one mole of CO2. The number of moles of methane converted is equal to the number of moles of electrons extracted from methane because each carbon atom in a methane molecule and each carbon atom in a carbon dioxide molecule. 119 moles of methane are being transformed in this
instance, hence 119 moles of electrons will likewise be lost. This happens because each carbon atom in methane loses four electrons during the oxidation process, resulting in the creation of one carbon dioxide molecule and the elimination of four moles of electrons.
to know more about respiration refer to the link below
https://brainly.com/question/22673336
#SPJ4
If I have ten eggs and lots of milk, flour and oil, how many
crepes can I make?
Answers
Answer:
I'd say about 8?
Explanation:
if you wanna make them huge make about 6
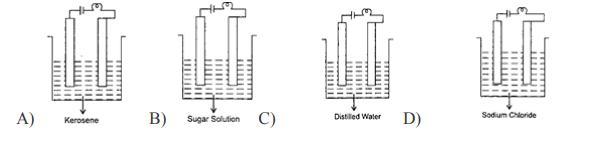
6. In which of the following will the bulb glow?
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
What questions might remain that will one day be answered by new technology?
Answers
Answer:
Can we find a safe cure for cancer?
Explanation:
pls mark brainliest
The modern quantum theory has which of the following as a model?
a)plum pudding
b)electron cloud model
c)planetary
d) nuclear
Answers
Answer:
B) electron cloud model
Explanation:
Because of the true nature of atom is actually look like a cloud model. The other three were before we observe a true atom
what kind of hybridization do you expect for each labeled carbon atom in the following molecules?
Answers
The type of hybridization for each labeled carbon atom in the given molecules depends on the number of sigma bonds and lone pairs around the carbon atom.
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energy levels. It helps explain the geometry and bonding in molecules. The hybridization of a carbon atom is determined by the number of sigma bonds and lone pairs around it.
Carbon atoms with 4 sigma bonds (no lone pairs) are sp3 hybridized. They have tetrahedral geometry, and the carbon atom's s orbital and three p orbitals hybridize to form four sp3 hybrid orbitals.
Carbon atoms with 3 sigma bonds and 1 lone pair are sp2 hybridized. They have trigonal planar geometry, and the carbon atom's s orbital and two p orbitals hybridize to form three sp2 hybrid orbitals.
Carbon atoms with 2 sigma bonds and 2 lone pairs are sp hybridized. They have linear geometry, and the carbon atom's s orbital and one p orbital hybridize to form two sp hybrid orbitals.
It's important to note that the determination of hybridization is based on the arrangement of sigma bonds and lone pairs around the carbon atom. The specific molecular structure and the presence of multiple carbon atoms in a molecule can affect the hybridization of individual carbon atoms. Therefore, a detailed analysis of the molecule's structure is necessary to determine the hybridization of each labeled carbon atom accurately.
Know more about carbon here:
https://brainly.com/question/13046593
#SPJ11
How many grams are there in 3. 4 x 1024 molecules of nh3.
Answers
Answer:
69,632 grams
explanation
number of molecules =mass\molemass
nh3 14 +2×3=20
3.4×1024=m/20
cross multiply
grams =69,632
Hi I need help!!!!! pleaseeeeeeeee!

Answers
Answer:
yes it chemical to electrical to radiant energy
Explanation:
correct