What are the 4 common elements that form covalent bonds?
Answers
Answer:
The four (4) most important elements found in cells that form covalent bonds are carbon, hydrogen, oxygen and nitrogen.
Explanation:
Related Questions
Guyton de Morveau, a French chemist, created a system for naming compounds that is still used today. For example, he said that a compound of zinc and chorine is called zinc chloride. Which of the
following is true about de Morveau's naming system?
A. The non-metallic atom is last.
B. The metallic atom is last.
C. The larger atom is first.
D. The smaller atom is first.
Answers
In de Morceau's nomenclature scheme, the smaller atom appears first.
What naming scheme is used?Nomenclature is an organism name system used in biological classification. Genus and species names, two Latinized nouns drawn from numerous sources, serve as indicators of the species to which the creature belongs.
Who created the current nomenclature for chemical substances?On August 26, 1743, Antoine-Laurent Lavoisier was born in Paris, France. He was a well-known French chemist and a key player in the 18th century chemical revolution. In addition to co-creating the current system for identifying chemical compounds, he established an experiment-based explanation of the chemical reactivity of oxygen.
To know more about nomenclature visit:-
https://brainly.com/question/25845195
#SPJ1
which ion is responsible for the solution being acidic or basic nac2h3o2
Answers
The ion responsible for the solution being basic or acidic in NaC2H3O2 is the Acetate ion.
The Acetate ion, CH3COO- is responsible for the solution being acidic or basic in NaC2H3O2.
NaC2H3O2 is also known as Sodium Acetate. It is a common compound in the laboratory that is colorless, deliquescent, and odorless. It dissolves easily in water, and its pH varies depending on the solution's acetate and acetic acid concentration.
Because acetate ion is a weak base, its solution has a higher pH than a solution containing just the acid. The buffer capacity of a solution of the salt NaC2H3O2 (Acetate ion) is dependent on the concentration of the salt and the pH of the solution. A solution with a pH of 7.0 and a 0.1 M NaC2H3O2 concentration would have a buffer capacity of 1.4. A solution with a pH of 5.0 and the same salt concentration would have a buffer capacity of 13.0.The equation for the dissociation of sodium acetate is given below:
NaC2H3O2 ⇌ Na+ + C2H3O2-
We can say that the solution is basic if the pH is greater than 7, acidic if the pH is less than 7, and neutral if the pH is equal to 7.
Hence, the Acetate ion is responsible for the solution being basic or acidic in NaC2H3O2.
learn more about dissociation here
https://brainly.com/question/16818822
#SPJ11
Please help me!
Thank you

Answers
Hope it helps!!
hiiiiii which class do u hate the most mine is math
Answers
Answer:
social studies
Explanation:
Classify the minerals into the different types of silicates.
a. Pyrope.
b. Tourmaline.
c. Actinolite.
d. Zoisite.
e. Enstatite.
f. Natrolite.
g. Talc.
Answers
Answer:
Silicate minerals are the most common of Earth's minerals and include quartz, feldspar, mica, amphibole, pyroxene, and olivine. Silica tetrahedra made up of silicon and oxygen, form chains, sheets, and frameworks, and bond with other cations to form silicate minerals.
Explanation:n
26-30. Why is it that a pitcher of orange juice flow smocthly when you transfer it to another container?
Answers
A pitcher of orange juice flow smoothly when you transfer it to another container because of lower viscosity value and large proportion of water in it.
What is viscosity?Viscosity is the resistance of a fluid to a change in shape or movement of portions as compared to one another. High viscous moves slowly as compared to less viscous.
So we can conclude that a pitcher of orange juice flow smoothly when you transfer it to another container due to lower viscosity.
Learn more about container here: https://brainly.com/question/11459708
The system below was at equilibrium in a
9.0 L container. What change will occur
for the system when the container is
shrunk to 3.0 L?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
Hint: How many moles of gas are on each side?
A. The reactions shifts to the right (products) to produce
fewer moles of gas.
B. There is no change because there are the same
number of moles of gas on both sides.
C. The reactions shifts to the left (reactants) to produce
more moles of gas.

Answers
The number of moles of gas is the same on both sides, the change in volume will not affect the equilibrium position of the reaction. The answer is B) There is no change because there are the same number of moles of gas on both sides.
To determine the change that will occur when the container is shrunk from 9.0 L to 3.0 L for the given reaction:
51.8 kJ + H₂(g) + I₂(g) → 2HI(g)
We need to consider the number of moles of gas on each side of the reaction.
On the left side, there are 2 moles of gas (H₂ and I₂), while on the right side, there are 2 moles of gas (2HI). Both sides have an equal number of moles of gas.
Therefore, the correct answer is B) There is no change because there are the same number of moles of gas on both sides.
For more details regarding the number of moles, visit:
https://brainly.com/question/20370047
#SPJ1
Common law larceny consisted of four distinct elements. Which element identifies the mens rea of larceny
Answers
The complete question is:
Common law larceny consisted of four distinct elements. Which element identifies the men's rea of larceny?
a. The trespassory taking
b. And carrying away
c. Of the personal property of another
d. With intent to permanently deprive the owner/professor of the property.
Larceny exists wrongfully taking and taking out another's belonging with the specific intent of:
(1) Permanently deprived of his owner
(2) Deny the owner of its control for an unreasonable length of time
(3) Utilize in a way that denies the owner of its value.
Therefore, the correct answer is option d. With intent to permanently deprive the owner/professor of the property.
What are the elements of larceny?Larceny exists wrongfully taking and taking out another's belonging with the specific intent of:
(1) Permanently deprived of his owner
(2) Deny the owner of its control for an unreasonable length of time
(3) Utilize in a way that denies the owner of its value.
The men's rea of larceny exists the intention to permanently deprive another of the property. There must be a concurrence between the purpose and the act. The purpose to borrow your neighbor's car exists not larceny.
Therefore, the correct answer is option d. With intent to permanently deprive the owner/professor of the property.
To learn more about larceny refer to:
https://brainly.com/question/12490184
#SPJ4
What is the name of cuo? explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
The name of CuO is Copper(II) oxide have the two most common oxidation states of copper are +2 and +1.
What is copper oxide used for?Copper oxide is a trace element for the zootechnical and agricultural sector. Cupric oxide is also used as a raw material for the production of catalysts and pigments in the field of ceramics, glass and plastics.
This compound makes two different types of bonds being the hydrogen bond when it's a hydrogen and covalent bond when we have an oxygen or a nitrogen.
See more about Copper(II) oxide at brainly.com/question/24217869
#SPJ1
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
how many grams of carbon are required to produce 75 L of CH4 (g) at STP
Answers
Answer: 62.4g according to a quizlet
Explanation:
the movement of carbon between the atmosphere, land, and ocean is called what?
Answers
solutions of the [v(oh2)6]3 ion are green and absorb light of wavelength 560 nm . calculate the ligand field splitting energy in the complex in units of kilojoules per mole.
Answers
The ligand field splitting energy of the [V(OH2)6]3+ complex is approximately 2.137 × 10^5 kilojoules per mole.
The ligand field splitting energy of the [V(OH2)6]3+ complex can be calculated using the equation ΔE = hc/λ, where ΔE is the energy difference, h is Planck's constant, c is the speed of light, and λ is the wavelength of light absorbed.
First, convert the wavelength to meters (560 nm = 560 × 10^(-9) m).
Then, substitute the values into the equation to calculate the energy difference.
Finally, convert the energy from joules to kilojoules per mole by multiplying by Avogadro's number and dividing by 1000. The resulting value will be the ligand field splitting energy of the complex in kilojoules per mole.
Using the equation ΔE = hc/λ, where h = 6.626 × 10^(-34) J·s (Planck's constant) and c = 2.998 × 10^8 m/s (speed of light), we can calculate the energy difference:
ΔE = (6.626 × 10^(-34) J·s × 2.998 × 10^8 m/s) / (560 × 10^(-9) m) = 3.548 × 10^(-19) J.
To convert this to kilojoules per mole, we need to multiply by Avogadro's number (6.022 × 10^23) and divide by 1000:
(3.548 × 10^(-19) J) × (6.022 × 10^23 mol^(-1)) / 1000 = 2.137 × 10^5 kJ/mol.
To learn more about ligand field click here
brainly.com/question/11856948
#SPJ11
can someone help me please ?

Answers
Answer:
5N would be the net force if i'm correct 5N and 5N cancle each other out then all your left with would be 5N
Explanation:
BTW whats your
name-
age-
and fav color just tring to meet new people
jing the scientist has one solution that is 30% acid and another solution that is 18% acid.
How much of each solution should she use to get 300 L of a solution that is 21% acid?
Answers
Jing the scientist has one solution that is 30% acid and another solution that is 18% acid. Solution that she should use to get 300 L of a solution that is 21% acid is 75L.
Anything with a pH lower than 7 is considered acidic. Hence, called as Acid.
Given,
300L of another solution with 21% acid is created by combining 30% of one solution with 18% of another solution.
Let x represent the proportion of solution 1 in the mixture.
Hence, another solution would be (300-x) L
According to given question,
30% of x + 18% of (300-x) = 21% of 300
i.e. 30x/100 + 18(300-x)/100= 21 × 300/100
=> 30x - 18x = 6300 - 5400
=> 12x = 900
=> x = 75L
Hence, 75L of each solution is required to get 300 L of a solution that is 21% acid.
Learn more about Acid here, https://brainly.com/question/24194581
#SPJ9
A mass of 33.6g of magnesium carbonate, MgCO3, completely decomposed when it was heated. It made 16.0 g of magnesium oxide, MgO Calculate the mass of carbon dioxide, CO2, produced in this reaction.
Answers
Answer:
17.6g
hahahahahahhaha
Much of the thermal energy within the Earth comes from atoms that decay. What is another major source of thermal energy within the
Earth?
A. gravitational energy left over from the formation of the Earth
B. thermal energy from the decay of dead plants and animals
c. thermal energy trapped by clouds and water vapor in the atmosphere
D. thermal energy absorbed by the Sun at the Earth's crust
Answers
Geothermal energy is heat produced by the Earth itself. It is a resource that may be gathered for human use and is renewable.
The correct option is (D) Thermal energy absorbed by the Sun at the Earth's crust
The friction and gravitational attraction that were produced when Earth was formed more than 4 billion years ago provide a modest amount of the core's heat. The continual production of heat on Earth, however, is mostly caused by the decay of radioactive isotopes like potassium-40 and thorium-232. From the surface to the core, the temperature of Earth increases with depth. The geothermal gradient is the term used to describe this progressive temperature variation. Rock that has partially melted is known as magma, which is gas- and bubble-filled. The lower crust and mantle both contain magma, which occasionally bubbles to the surface as lava.Nearby rocks and subsurface aquifers are heated by magma. Geysers, hot springs, steam vents, undersea hydrothermal vents, and mud pots are among ways that hot water can be emitted.They are all powered by geothermal energy. Their heat may be captured and used directly for heating, or their steam can be used to generate electricity.Learn more about the Geothermal energy with the help of the given link:
https://brainly.com/question/1061324
#SPJ1
One of the biggest news stories of 1996 was the successful cloning of Dolly the sheep. Dolly was the first mammal cloned from an adult body cell. At first, Dolly appeared to be perfectly healthy. However, she died at age 6 of cancer. Dolly’s early death made scientists wonder whether cloned animals age faster than normal. A small flock of sheep cloned from Dolly have been observed since 2007. Happily, these clones show no sign of early aging. Choose the best option to complete the sentence. Scientists worried that cloned animals would age rapidly because their cells contain __________________ as old as the individual they were cloned from.
Answers
Answer:
I believe the answer you're looking for is DNA, not sure though
what happens if you don't disconnect the wires in a series circuit?
Answers
Answer:
the series circuit has one current so if you don't disconnect the wires nothing happens
but if you disconnect the wires in series circuit, the current stop and the current doesn't go to the end
Explanation:
(≧▽≦)hope it helps (≧▽≦)
Column (A)
1. Mercury
2. Oxygen (
3. Bromine
Bd
4. Mercuric oxide
5 silver b
6. Chemical change
Column (B)
a) decomposition of a
compound
b) malleable and
ductile
c) nonmetal, liquid at
room temperature
d) compound
e) metal, liquid at room
temperature
f) does not have a
definite volume
Answers
1. mercury - metal, liquid at temperature
2. oxygen - does not have a definite volume
3.Bromine - non-metal, liquid at room temperature
4.mercuric oxide - compound
5. silver -malleable and ductile
6. chemical change - decomposition of compound
What is mercury?The only common metal that is liquid at room temperature is mercury, a chemical element. It is a thick, silvery-white liquid metal that is frequently referred to as quicksilver. Mercury is a metal in transition.
Since mercury is a chemical element, it cannot be made or destroyed. Since the creation of the earth, the same quantity has been present on the planet. However, both natural and human activity can cycle mercury in the environment. Additionally, there have been numerous new uses for mercury metal in electrochemistry and electrical equipment. Mercury metal has a significant vapour pressure at normal temperature and is a volatile liquid.
At 20°C, mercury has a density of 13.6 g.cm-3.
Mercury is a metallic element that is liquid and has a wide range of uses. Additionally, it has dangerous qualities.
Learn more about mercury here:
brainly.com/question/585198
#SPJ13
Which of the following has the least elastic potential energy?
A: a fully stretched rubber band
B: a rubber band sitting on a table
C: a rubber band pulling hair back
Answers
Answer:
B
Explanation:
B.
The rubber band is not being stretched which does not create any potential energy storage.
Which statement best explains the temperatures of a desert environment? A. The high amount of moisture in the air traps heat causing extreme temperature differences. B. The high amount of moisture in the air traps heat causing a very stable temperature range. C. The lack of moisture in the air allows heat to escape quickly at night causing a very stable temperature range. D. The lack of moisture in the air allows heat to escape quickly at night causing extreme temperature differences.
Answers
The high amount of moisture in the air traps heat causing extreme temperature differences.
A narrow range of temperatures allows organisms to survive. It varies between five degrees Celsius and thirty-five degrees Celsius. It best describes a desert climate with warm to hot temperatures year-round without a rainy season.
The desert environment is so dry that it supports only very sparse vegetation. There are few trees and shrubs or herbaceous plants that only form a very incomplete ground cover under normal climatic conditions. Deserts form when there is a lack of sunlight due to a lack of moisture. Evaporation is less due to the relatively low water content. There are also fewer clouds reflecting sunlight.
Learn more about A desert environment here:-https://brainly.com/question/3363067
#SPJ1
Answer:
d
Explanation:
Iodine- 131 Is a radioactive isotope, and is often used in certain medical treatments. It has a short half life of about 8 days. It a hospital has a 1050 mg sample of it’s available, how much would be absolute after 72 days
Answers
After 72 days, a 1050 mg sample of Iodine-131 would have decayed to approximately 46.87 mg.
What is a half-life and how does it relate to the decay of radioactive isotopes?A half-life is the time it takes for half of a sample of a radioactive isotope to decay. It is a characteristic property of each isotope and can be used to predict how long it will take for a given sample to decay to a certain amount. The amount of an isotope remaining after a certain amount of time can be calculated using the half-life and the formula N = N0 * (1/2)^(t/T).
Why is Iodine-131 used in medical treatments and how does its short half-life factor into its use?Iodine-131 is used in medical treatments because it emits beta particles that can destroy cancerous cells. Its short half-life is an advantage because it allows for a higher dose of radiation to be delivered to the tumor while minimizing the amount of radiation exposure to healthy tissues. After a few weeks, the Iodine-131 decays to a negligible amount and the patient is no longer radioactive.
To know more about radioactive isotopes,visit:
https://brainly.com/question/1907960
#SPJ1
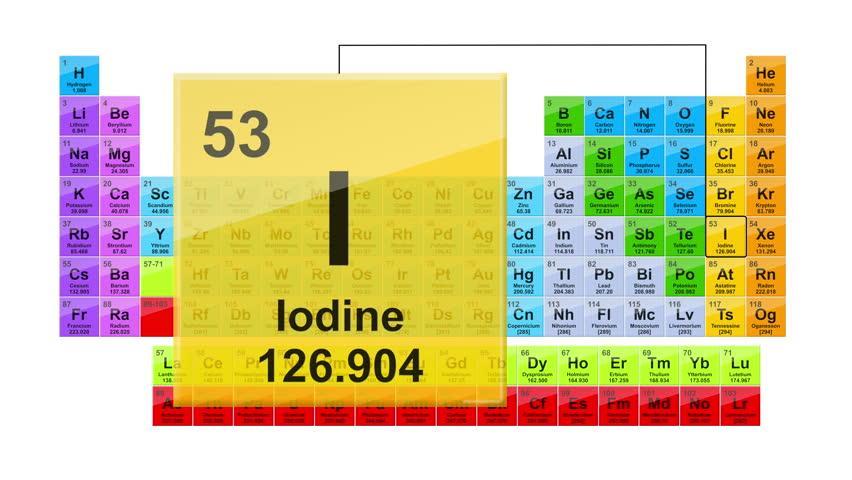
Which element is in Group 17 and has more than 50 protons but less than 75 protons?
Answers
Answer:
Iodine
Explanation:
Iodine is in group 17 on the periodic table (first picture attached). The second picture shows you in the top left-hand corner of Iodine's little square, there is the number 53. This is the atomic number, it is also the number of protons and electrons in an element.
Hope this helped :)

What a bat hits a ball what is the impulse
Answers
Answer:
Plugging in the numbers we find the average force to be Favg=18,436 N, which is equivalent to 4124 lbs of force. The impulse delivered by this force is the product of the average force the the contact time, resulting in an impulse of 12.91 Ns.
Answer:
the force multiplied by the time the objects are in contact
Explanation:
took the quiz
Which of the following is an aldehyde?

Answers
Answer: \(CH_3CH_2CH_2CHO\) has aldehyde functional group.
Explanation:
Functional groups are specific group of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules.
A. \(CH_3CH_2CH_2COCH_3\) has ketone \(C=O\) functional group .
B. \(CH_3CH_2CH_2COOH\) has carboxylic acid \(COOH\) functional group .
C. \(CH_3CH_2CH_2CHO\) has aldehyde \(H-C=O\) functional group .
D. \(CH_3CH_2CH_2COOCH_3\) has ester \(RO-C=O\) functional group .
Thus \(CH_3CH_2CH_2CHO\) has aldehyde (CHO) group.
When kc decreases what happens to the molecules in terms of speed space and attractive force pls help
Answers
Kc decreases as the temperature in molecules increase.
What conclusion did Rutherford draw from his gold-foil experiment? A. Almost all the mass of an atom is concentrated in the nucleus. B. Atoms contain three different subatomic particles. C. Electrons are tiny particles that carry a negative charge. D. The mass of a proton is nearly equal to the mass of a neutron.
Answers
Answer:
A. Almost all of the mass is concentrated in the nucleus.
Explanation:
Because when he shot the alpha particles towards the atoms, most passed through (which meant atom is mostly empty space), but some bounced back and such (which meant mass is concentrated in the nucleus.)
The reason they bounced is because alpha particles are postive and nucleus is positive as well, and we know positives don't attract, rather they repel thus they bounced.
a bicyclist travels 15 km over 2.0 hours of travel. Calculate the bicyclist's average speed in km/h
Answers
Answer:
7,5km/h
Explanation:
Average speed = total distance travelled/time taken
Average speed = 15/2
Average speed = 7,5km/h
(This should be in physics/maths not Chemistry)
Which effect results when a solute dissolves in a solvent? *
lowering of the boiling point
elevation of the vapor pressure
lowering of the freezing point
invariable lowering of solution temperature
elevation of the melting point
Answers
Answer:
The temperature of the solution will decrease.
Explanation:
Less energy is released than is used, the molecules of the solution move more slowly, making the temperature decrease.
According to the concept of solubility, lowering of the freezing point results when a solute dissolves in a solvent.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance usually a solvent. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute which is present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on parameters of temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/28170449
#SPJ2