What are the elements that make up the compound, and what are some properties of these elements?
Answers
Answer: Below (=
Explanation:
The properties of a compound depend not only on which atoms the compound contains, but also on how the atoms are arranged. Atoms of carbon and hydrogen, for example, can combine to form many thousands of different compounds.
Related Questions
Where do convection currents occur?
A.
In areas with the same temperature
B.
In areas with different air pressures
C.
In areas with the same altitude
D.
In areas with different cloud types
Answers
Answer:
a because where the air travels for example a radiator
Answer:
C.
In areas with the same altitude
Explanation:
What is the shorthand electron configuration of a potassium ion (K+)?
Answers
The shorthand electron configuration of a potassium ion (K+) is [Ar] 4s1.
Electron configuration refers to the arrangement of electrons in an atom or ion. In a neutral atom, the number of electrons is equal to the number of protons, resulting in a neutral charge. However, in an ion, the number of electrons is not equal to the number of protons, resulting in a charge.
The shorthand electron configuration uses symbols from the periodic table to represent the elements, with electron configurations being abbreviated by using the noble gas symbol (in square brackets) that precedes the element in question in the periodic table. In this case, the noble gas symbol [Ar] represents Argon, and the electron configuration of K+ is then given as [Ar] 4s1, indicating that the electron configuration of potassium has lost one electron from its 4s orbital, leaving a single electron in the 4s orbital and a positive charge on the ion.
In more detailed terms, the electron configuration of a neutral potassium atom would be [Ar] 4s1 3d10, where the [Ar] represents the electron configuration of Argon (the preceding noble gas in the periodic table), and the 4s1 and 3d10 indicate the number of electrons in the 4s and 3d orbitals, respectively. However, in the case of a potassium ion, one electron has been removed, resulting in a positive charge and the shorthand electron configuration of [Ar] 4s1.
Here you can learn more about electron configuration
https://brainly.com/question/30257751#
#SPJ11
i need help lol, i need this done as soon as possible just the chart, and i will GIVE brainlist
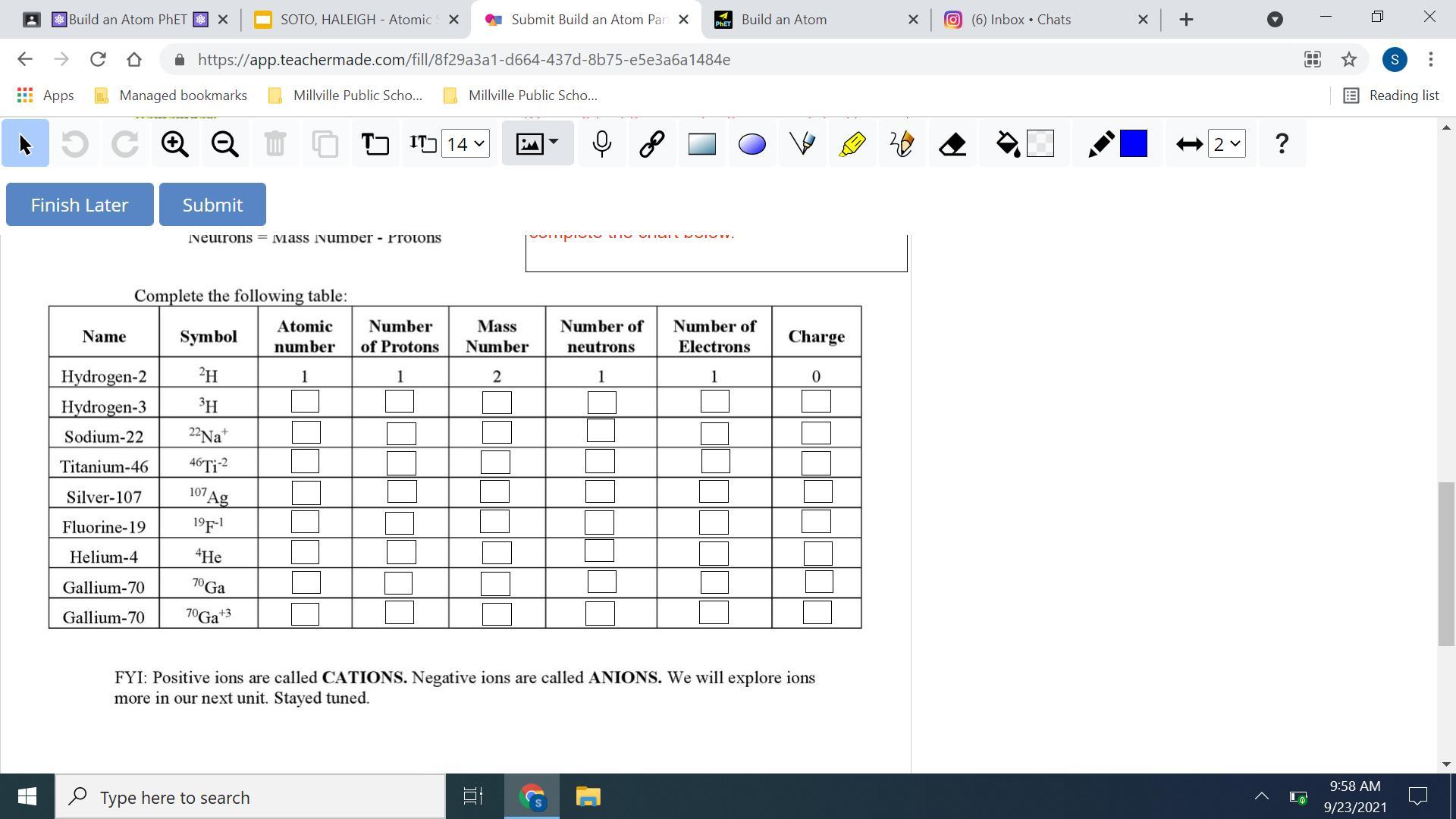
Answers
determine the percent yield of a reaction that produces 22.6 g of fe when 50.00 g of fe2o3 react with excess al according to the following reaction. Molar Mass Fe2O3 = 159.7 g/mol
Answers
The percent yield of the reaction is 82%
First step: make the chemist equation.
2 Al (s) + Fe2O3 (s) → 2 Fe (s) + Al2O3 (s)
As the statement says that aluminium is in excess, the limiting reactant is the Fe₂O₃.
Second step: Find out the moles in the reactant.
Molar weight Fe2O3: 159.7 g/m
Mass / Molar weight = moles
50 g /159.7 g/m = 0.313 moles
Third step: Analyse the reaction. 1 mol of Fe2O3 makes 2 moles of Fe.
1 mol Fe2O3 ____ 2Fe
0.313 mol Fe2O3 ____ 0.626 moles
Molar weight Fe = 55.85 g/m
Moles . molar weight = mass
55.85g/m . 0.626m = 34.9 grams
This will be the 100% yield of the reaction but we only made 22.6 g
34.9 g ____ 100%
22.6 g ____ 64.75 %
Hence, the percent yield is 64.75 %.
Learn more about percent yield from the link given below.
https://brainly.com/question/14903519
#SPJ4
Soap has an ionic and a polar end. It works well to remove oil by
1)surrounding the oil and water with the polar end.
2)surrounding the oil with the nonpolar end, and the water interacts with the polar end.
3)surrounding the oil with the polar end, and the water interacts with the nonpolar end.
4)surrounding the oil and water with the nonpolar end.
Answers
Soap has both ionic and polar ends, which allows it to effectively remove oil. It works by surrounding the oil with its nonpolar end, and the water interacts with the polar end.
Soap molecules have both a polar and nonpolar end, making them amphipathic. The nonpolar end is hydrophobic and interacts with nonpolar substances such as oil, while the polar end is hydrophilic and interacts with water.
When soap is added to a mixture of oil and water, the nonpolar end of the soap molecules surround the oil droplets, while the polar end interacts with the water.
This creates small clusters of oil and soap molecules called micelles, which are suspended in the water.
The micelles allow the oil droplets to be more easily removed from the surface being cleaned, as they are surrounded by the hydrophilic polar ends of the soap molecules and can be washed away with water.
Visit here to learn more about molecules:
brainly.com/question/475709
#SPJ11
what is the mass of potassium chloride when 2.50 g of potassium reacts with excess of chlorine gas
Answers
Answer:
4.52 grams of potassium chloride
Explanation:
First you need a balanced reaction equation K(s) + Cl₂ (g) --> 2 KCl(s). Since the chlorine gas is said to be in excess all of the potassium will be converted to form potassium chloride. Convert grams of potassium to moles of potassium with the periodic table, then you can convert moles of potassium to moles of potassium chloride with the balanced equation, finally convert moles of potassium chloride to grams of potassium chloride with the periodic table. Set up a dimensional analysis chart to help with conversions
(2.50 grams K) * (1 mole K) * (2 mole KCl) * (35.45 grams KCl)
(39.09 grams K) (1 mole K) (1 mol KCl)
Which equals 4.52440778 grams, with sig figs it should be 4.52 grams of potassium chloride
Lets say a giant radioactive monster is attacking
Phoenix. This monster is so radioactive and big the air in
Feels easily over 120°. Is this an endothermic or
exothermic reaction?
Answers
Answer: exothermic reaction
The monster is releasing heat energy to the environment surrounding it. The term "exo" means "outside".
In contrast, an endothermic reaction is one where energy is absorbed from the environment to cool down the surrounding air.
Drag each label to the correct location in the equation. Not all tiles will be used. The density of mercury is 13. 6 grams per cubic centimeter. Complete the steps for converting 13. 6 g/cm3 to kg/m3. (1 kg = 1,000 g, 1 m3 = 106 cm3).
Answers
The Complete the steps for converting the density of mercury is
13.6 g x 1 Kg x 10⁶ cm³ = 13600Kg
cm³ 1000g m³
the method of changing 13.6 g/cm3 to kg/m3
A kilogram is equal to one thousand grams.
Consequently, it may be written as
1 Kg = 1000g
1g = 1kg
1000
Therefore, 1 kg will be entered into the first blank (numerator).
Currently, 100 centimeters make up 1 meter.
Thus,
1 m³ = (100)³ cm³
1cm³ = 1m³
10⁶
so the second blank (numerator). will be filled with 10⁶
Additionally, the third blank will be filled with 1 m³
And 13600 will be the final blank.
The final equation will look like this:
13.6 g x 1 Kg x 10⁶ cm³ = 13600Kg
cm³ 1000g m³
Your question is incomplete but most probably your full question attached below
learn more about density here:
brainly.com/question/26364788
#SPJ4

SEP Interpret Data The table shows the atomic radi and balline
points of five halogens that experience intermolecular disea
forces. Plot the boiling point vs the atomic radius was the
resulting pattern to predict the boiling point
of astatine.
Answers
The table shows that as the atomic radius grows, the boiling points of the halogens rise. This implies that the intensity of the intermolecular interactions between the atoms and the size of the halogen atom are related.
Because astatine has a bigger atomic radius than iodine, we can infer from this pattern that it will have a higher boiling point.
Atomic radiusThe attractive forces that occur between molecules are known as intermolecular forces. The sort of molecules involved, as well as their size, shape, and polarity, all affect how strong these forces are. Intermolecular forces are often stronger for bigger molecules and molecules with more polarity.The cause of this pattern is that the distance between the atoms in a molecule rises along with the size of the halogen atom. The London dispersion forces between the molecules become stronger as a result. These forces are a result of the transient dipoles formed as electrons move about in the atom's or molecule's electron cloud.learn more about boiling point here
https://brainly.com/question/40140
#SPJ1
Please help and right down each step!!

Answers
The mass of \(Ag_2CrO_4\) that would be formed will be 2.69 grams.
Stoichiometric problemMole of 2.5 grams silver nitrate = 2.5/143.32 = 0.017 mol
Mole of 18.0 mL, 0.450 M sodium chromate = 18/1000 x 0.450 = 0.0081
From the balanced equation of the reaction, the mole ratio of silver nitrate to sodium chromate is 2:1. In other words, the sodium chromate is a bit limiting.
The mole ratio of sodium chromate and \(Ag_2CrO_4\) is 1:1. Hence, the equivalent mole of \(Ag_2CrO_4\) formed would also be 0.0081 mol.
Mass of 0.0081 mol \(Ag_2CrO_4\) = 0.0081 x 331.73
= 2.69 grams
In other words, the mass of \(Ag_2CrO_4\) formed from the reaction would be 2.68 grams.
More on stoichiometric problems can be found here: https://brainly.com/question/29856106
#SPJ1
Từ hóa trị của Cl trong hợp chất HCl, hãy lập công thức hóa học của hai hợp chất do kim loại K, Ca liên kết với Cl
Answers
Answer:
kcl,cacl2
Explanation:
The evidence of chemical changes with an example of each (include all 5)
Answers
Answer:
??????????
Explanation:
PROCEDURAL NOTE: The baby was placed on a standard circumcision board. He was prepped in the standard procedure with Betadine. We then used sucrose and a pacifier. 0.5 cc of lidocaine was injected at 20 ′
clock and 10 o clock. He tolerated the procedure well. We then used a Gomco clamp and removed the foreskin. Vaseline gauze was applied. There were no complications. 1. CPT Code: 2. ICD-10-CM Code: ⋆⋆ (N47.1). This code would be used whether it is congenital or acquired. There are no fourth or fifth digits to assign."
Answers
The CPT code for circumcision using a clamp is 54150. This code is used for the circumcision of a 2-week-old male infant. The CPT code for Encounter for circumcision and for other male genital surgery is Z41. 0.
The International Classification of Diseases, tenth revision, Clinical Modification (ICD-10-CM) is a classification system used by doctors and other healthcare professionals to classify all diagnoses, symptoms, and procedures recorded in connection with hospital care.
It provides the level of detail required for diagnostic specificity and classification of morbidity in the United States.
To learn more about CPT code, refer to the link:
https://brainly.com/question/30410104
#SPJ4
What is different about the rotation of Uranus?
the axis is vertical
there is no axis of rotation
the axis is pointing toward the sun
Answers
Answer:
Unlike the other planets of the solar system, Uranus is tilted so far that it essentially orbits the sun on its side, with the axis of its spin nearly pointing at the star. This unusual orientation might be due to a collision with a planet-size body, or several small bodies, soon after it was formed.
Explanation:
the axis is vertical
Answer:
The axis is pointing towards the sun!!!
Explanation:
Choose all of the following that are FALSE.
A. Paper makes up the largest proportion of MW in the United States.
B. If you wash your plastic bottles with warm water that was heated via coal-generated electricity before recycling them, then recycling your plastic bottles could release more carbon into the atmosphere than throwing them
away.
C. Total waste generation in the United States has been steadily increasing since about 1950. Globally. D. solid waste management costs are expected to begin decreasing as waste management
technology gets cheaper.
Answers
A. Paper makes up the largest proportion of MW in the United States. (False) C. Total waste generation in the United States has been steadily increasing since about 1950. Globally. (False)
D. Solid waste management costs are expected to begin decreasing as waste management technology gets cheaper. (False)
The false statements are A, C, and D.
A. Paper does not make up the largest proportion of municipal waste (MW) in the United States. While paper waste is significant, it is not the largest component. Other materials like food waste, plastics, and metals also contribute to MW.
C. Total waste generation in the United States has not been steadily increasing since about 1950. In fact, waste generation rates have fluctuated over the years due to various factors such as population growth, consumption patterns, and waste management practices.
D. Solid waste management costs are not expected to decrease as waste management technology gets cheaper. While advancements in technology can lead to more efficient waste management processes, they often come with their own costs, such as implementation, maintenance, and regulatory compliance. These factors can offset any potential cost savings and may even lead to an increase in waste management costs over time.
learn more about Solid waste management here:
https://brainly.com/question/14665452
#SPJ11
Aditya Birla Cement Manutacturing Company manufactures cement for use in construction of stone builelings. Beginning work in process inclustes 400 urvits that are 20% compiete with respect to conversion and 30% complete with respect to materials. Ensing work in process inclades 200 units that are 40% complete with respect to corversion and 50 E complete with respect to materials, 2,000 units were stated duting the perlod. Also, assume that $9,900 of material costs and $14,880 of cortversion costs were in the beginning inventory and $180,080 of materials and $409,200 of conversion costs were added to paoduction duing the period. What is the total cost pet equivalent unit using the weighted average method? Multiple Choice $26860 $26785 578000 $26500
Answers
The correct option is $26785.To calculate the total cost per equivalent unit using the weighted average method, we need to consider the costs incurred in both the beginning work in process and the units added during the period.
First, let's calculate the equivalent units of production for both conversion and materials:
Conversion costs:
Beginning work in process: 400 units × 20% complete = 80 equivalent units
Units added during the period: 2,000 units × 40% complete = 800 equivalent units
Total equivalent units for conversion = 80 + 800 = 880 equivalent units
Material costs:
Beginning work in process: 400 units × 30% complete = 120 equivalent units
Units added during the period: 2,000 units × 50% complete = 1,000 equivalent units
Total equivalent units for materials = 120 + 1,000 = 1,120 equivalent units
Next, let's calculate the total costs incurred:
Conversion costs:
Beginning work in process cost: $14,880
Costs added during the period: $409,200
Total conversion costs = $14,880 + $409,200 = $424,080
Material costs:
Beginning work in process cost: $9,900
Costs added during the period: $180,080
Total material costs = $9,900 + $180,080 = $189,980
Now, we can calculate the total cost per equivalent unit:
Total cost per equivalent unit = (Total conversion costs + Total material costs) / (Total equivalent units for conversion + Total equivalent units for materials)
Total cost per equivalent unit = ($424,080 + $189,980) / (880 + 1,120)
Total cost per equivalent unit ≈ $267.85
Therefore, the correct option is $26785.
To know more about conversion and materials, click here, https://brainly.com/question/1162213
#SPJ11
Could you use the method of water displacement to determine the volume of wood? Why?
Answers
Yes, you can use the method of water displacement to determine the volume of wood. The reason is that the water displacement method allows you to measure the volume of irregularly shaped objects, like wood, by observing how much water is displaced when the object is submerged.
Here are the steps to use water displacement for measuring the volume of wood:
1. Fill a container with enough water to submerge the wood completely.
2. Note the initial water level.
3. Carefully submerge the wood in the water, making sure no air bubbles are trapped.
4. Note the change in the water level.
5. Calculate the difference between the initial and final water levels. This difference represents the volume of the wood, as the amount of water displaced is equal to the volume of the object submerged.
Learn more about volume.
brainly.com/question/1578538
#SPJ11
which of the following groups tends to be overrepresented in the electorate?
Answers
Individuals with a high level of education tend to be overrepresented in the electorate. Here option D is the correct answer.
This is because higher levels of education are often associated with higher levels of political engagement and knowledge, and therefore a greater likelihood of participating in elections. In addition, individuals with higher levels of education are more likely to have access to the resources and information needed to register to vote and to navigate the voting process.
However, it is important to note that the overrepresentation of highly educated individuals in the electorate can have implications for the political system as a whole. This may result in policies that are more favorable to the interests of highly educated individuals, potentially at the expense of other groups.
It is therefore important to strive for a more diverse and representative electorate, in order to ensure that the voices and interests of all citizens are heard and taken into account in the political process.
To learn more about political engagement
https://brainly.com/question/30781303
#SPJ4
Complete question:
Which of the following options describes a group that tends to be overrepresented in the electorate?
A) Young adults
B) Low-income earners
C) Non-white ethnic groups
D) Individuals with a high level of education
a student uses a glue stick with an area of 4 cm3, putting
a pressure of 0.5 N/cm2 on her book. Calculate the force
she puts on the glue stick.
Answers
Answer:
So F=2N
Hope this helps.
Explanation:
P= F/A, where P is pressure, F is force, A is area.
So
P=F/A
0.5N/cm2 = F/4cm2 <--(do cross
2N=F multiplication,
4×0.5)
( And pls check on the unit of area u wrote, it should be (4cm2), not (4cm3) Unit of area is cm2.)
1. in this experiments you observed that the colors of the flames in each sample are different. why are all the flames not the same colors
Answers
The colors of flames in experiments can vary based on several factors, including the temperature and composition of the burning material.
In general, the color of a flame is determined by the emission spectrum of the excited molecules and atoms in the flame. When a material is burned, the heat and energy generated excites the molecules and atoms, causing them to emit light. The specific colors that are emitted depend on the temperature of the flame, as well as the chemical composition of the burning material.
For example, in a very hot flame, such as the flame produced by a welder's torch, the temperature can be high enough to excite and ionize the atoms of the burning material, causing them to emit light across the entire visible spectrum and beyond. This results in a flame that appears white.
In cooler flames, such as those produced by a candle, the temperature is not high enough to ionize the atoms, but it is still high enough to excite them and cause them to emit light. The specific colors that are emitted in this case depend on the chemical composition of the burning material. For example, in a candle flame, the wax is primarily composed of hydrocarbons, which emit a yellow-orange light when burned. The blue color that is often seen in the center of the flame is due to the reaction between the hydrogen and carbon in the wax, which produces excited molecules that emit blue light.
In conclusion, the colors of flames in experiments can vary based on the temperature and composition of the burning material. The specific colors that are observed depend on the conditions within the flame and the chemical reactions that are taking place.
Here you can learn more about the colors of the flames
https://brainly.com/question/23955162#
#SPJ11
I’m kind of confused.

Answers
The experimental rate law is; Rate = k[O2] [NO]^2
Thus the reaction is first order with respect to oxygen and second order with respect to nitrogen monoxide
What is the rate constant?The rate constant, represented by the symbol k, is a proportionality constant that relates the rate of a chemical reaction to the concentration of reactants. The rate constant is specific to a particular reaction and is determined experimentally.
The rate law of a chemical reaction expresses the relationship between the rate of the reaction and the concentrations of the reactants.
We know that;
2.56 * 10^-1/8.52 * 10^-2 = (0.09/0.03)^n
3 = 3^n
n = 1
Again;
7.67 * 10^-1/8.52 * 10^-2 = 0.06/0.02
9 = 3^n
3^2 = 3^n
n = 2
The rate law is;
Rate = k[O2] [NO]^2
Learn more about rate law:https://brainly.com/question/30379408
#SPJ1
A big ______ shows up on a weather map as really close/tight isobars & in weather as fast, strong winds
dewpoint
air pressure gradient
precipitation
barometer
Answers
Answer:
A BIG DI*CK
Explanation:
Rx Ephedrine sulfate (fz. pt = -0.13°C). 2%
Sodium chloride MW 58.5
Purified water qs ad. 30 mL
How much sodium chloride should be used to make this eye
solution isotonic with tears?
the answer is 22
Answers
The correct answer is the amount of sodium chloride needed to make this eye solution isotonic with tears is approximately 1.85 grams. Rounding it up to the nearest whole number gives us the answer as 2. Hence, the correct option is 22.
The given solution is a hypotonic solution as the solution's tonicity is lower than that of the tears. The tears contain 0.9% w/v of NaCl, which is isotonic with tears. So, to make the given solution isotonic, the amount of sodium chloride needs to be added.
The concentration of NaCl in tears is 0.9% w/v. Additional Information: We know that % w/v is the amount of solute present in grams per 100 ml of the solution. Therefore, 0.9% w/v means 0.9 grams of NaCl is present in 100 mL of tears.
To make 30 ml of isotonic solution, we can use the following formula: Equivalent weight of NaCl = 58.5/2 = 29.25 (as NaCl ionizes to give Na+ and Cl- ions)Moles of NaCl required to make 30 ml isotonic solution = 0.9 × 30 / 1000 = 0.027Moles of Na+ and Cl- ions present in 30 mL of isotonic solution = 2 × 0.027 = 0.054
A number of grams of NaCl needed to prepare 30 mL of isotonic solution is calculated as follows:0.054 g = (0.027 x 29.25 x X) / 1000Where X is the amount of NaCl required to make 30 mL isotonic solution. Solving this equation gives us: X = 1.85 g (approx). Therefore, the amount of sodium chloride needed to make this eye solution isotonic with tears is approximately 1.85 grams. Rounding it up to the nearest whole number gives us the answer as 2. Hence, the correct option is 22.
know more about hypotonic solution
https://brainly.com/question/122954
#SPJ11
whose main job is to break down sugar to release energy that a plant cell can use?
Answers
Answer:
The Mitochondria
Explanation:
Mitochondria are membrane-bound cell organelles that generate most of the energy required to power the cell's organic chemistry reactions. Energy created by the mitochondria is kept in a tiny molecule known as adenosine triphosphate (ATP).
If you know the atomic number and mass number of an atom of an element, how can you determine the number of protons, neutrons, and electrons in that atom?
Answers
Answer:
You subtract the atomic number from the mass number to find the number of neutrons. If the atom is neutral, the number of electrons will be equal to the number of protons.
i still need a bit of help.

Answers
If you added a proton to an atom of aluminum it would become a silicone ion
Why can the liquid metal be poured into a block shaped mold?
Answers
Answer:
Metal casting makes use of the way solids can melt into liquids and freeze back into solids.
ultiple qualitative tests can be used to determine the properties of carbohydrate samples. identify the test that provides the given information about carbohydrates. identify reducing sugars choose... distinguish between monosaccharides and disaccharides choose... distinguish between a pentose and a hexose choose... determine whether starch is present
Answers
1. To identify reducing sugars, use the Benedict's test.
2. To distinguish between monosaccharides and disaccharides, use the Barfoed's test.
3. To distinguish between a pentose and a hexose, use the Seliwanoff's test.
4. To determine whether starch is present, use the Iodine test.
1. Benedict's test: This test detects the presence of reducing sugars, which have free aldehyde or ketone groups. When heated with Benedict's reagent, reducing sugars react and produce a color change ranging from green to red-orange, depending on the sugar concentration.
2. Barfoed's test: This test differentiates monosaccharides from disaccharides. When heated with Barfoed's reagent, monosaccharides react quickly and form a red precipitate, while disaccharides react more slowly or not at all.
3. Seliwanoff's test: This test is used to distinguish between pentoses and hexoses. When heated with Seliwanoff's reagent, pentoses produce a red color, while hexoses produce a yellow color.
4. Iodine test: This test detects the presence of starch. When iodine solution is added to a sample containing starch, the solution turns a blue-black color.
By using the Benedict's, Barfoed's, Seliwanoff's, and Iodine tests, you can identify reducing sugars, distinguish between monosaccharides and disaccharides, differentiate between pentoses and hexoses, and determine the presence of starch in carbohydrate samples.
For more information on test for carbohydrates kindly visit to
https://brainly.com/question/29655942
#SPJ11
Assume we have 759 liters of N, at ST. What is the mass of the nitrogen gas? Give answers to the nearest whole number.
Answers
So, at STP, there are around 891 grammes of nitrogen gas in every 759 litres.
Which mass is greater, 14 or 15?The two stable isotopes of naturally occurring nitrogen (7N) are nitrogen-14 and nitrogen-15, with nitrogen-14 constituting 99.6% of all naturally existing nitrogen. Along with one nuclear isomer, 11mN, fourteen radioisotopes with atomic masses ranging from 10 to 25 are also known.
Assuming "ST" refers to standard temperature and pressure (0°C and 1 atm), we can use the ideal gas law to calculate the mass of nitrogen gas:
PV = nRT
where n is the number of moles, P is the pressure, V is the volume, and T is the temperature, and R is the gas constant.
The temperature and pressure are 273.15 K and 1 atm, respectively, at STP.
To solve for n, the number of moles, we can rearrange the ideal gas law equation as follows:
n = PV / RT = (1 atm) * (759 L) / (0.0821 L·atm/(mol·K) * 273.15 K) = 31.8 mol
Now we can calculate the mass of nitrogen gas:
mass = n * molar mass = 31.8 mol * 28.01 g/mol ≈ 891 g.
To know more about nitrogen gas visit:-
https://brainly.com/question/13907528
#SPJ1
which of the following is not a network solid? a. elemental silicon, si(s) b. diamond, c(s) c. co2(s) d. silicon dioxide, sio2(s) e. graphite
Answers
The option (c) is correct - C60(s).
What is network solid?
Atoms are covalently bound together to form layers of two-dimensional networks or a three-dimensional network in covalent network solids. Covalent network solids have high melting points because of the covalent bonds' strength.
What is solid ?
solid ground: a substance that is hard, firm, or compact. relative firmness, particle coherence, or form permanence in matter that is not a liquid or a gas, such as solid particles suspended in a liquid. regarding such a subject: Ice is water that has solidified.
Remaining all are network solids.
Elemental silicon is also a network solid, because its structure is very similar with the structure of diamond which is a network solid.
The fullerenes is allotrope of carbon which is not consider as network solids because of its structure curved into a ball.
Therefore, option (c) is correct - C60(s).
Learn more about network solid from the given link.
https://brainly.com/question/27657808
#SPJ4