What are the number of atoms in chromium
Answers
Answer: The correct answer is 24
Explanation:
Related Questions
Write in a scientific notation 234.5398
Answers
Answer:
the answer is 2.34 x 10^2
Compared with halogens, the alkali metals in the same period has
electronegativity
the same
O larger
O smaller
Answers
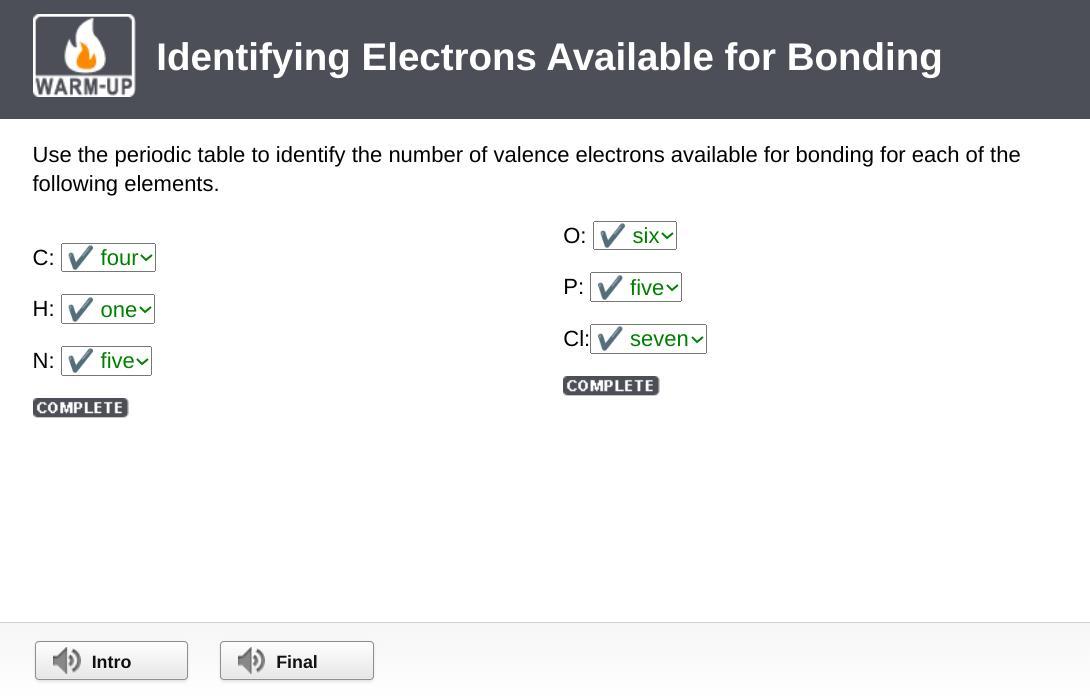
Use the periodic table to identify the number of valence electrons available for bonding for each of the following elements.
Answers
Answer: Edgy, 2020
Explanation: <3

Answer:
refer to picture below :)
Explanation:
When are atoms considered to be stable?
Answers
Answer:
Atoms are at their most stable when their outermost energy level is either empty of electrons or filled with electrons.
The mass number of a chromium atom is 52 and it has 24 protons. How many neutrons does this atom have? 24 28 76 80
Answers
The number of protons and neutrons together makes the total atomic mass of the element. The atom of chromium has 28 neutrons. Thus, option b is correct.
What is an atomic mass?An atomic mass is the property of an element that defines the number of protons and neutrons of the atom placed in a periodic table. The atomic mass is represented at the lower half of the atomic symbol.
The atomic mass is the sum of the neutrons and the protons that are held together in the nucleus of the atom as a concentrated mass. The atomic number is given as,
Atomic number = number of protons + number of neutrons
Given,
The atomic mass of chromium = 52
Number of protons = 24
Substituting values above:
52 = 24 + number of neutrons
number of neutrons = 52-24
= 28
Therefore, the number of neutrons of a chromium atom is 28.
Learn more about atomic mass here:
https://brainly.com/question/17067547
#SPJ6
Answer: option b, 28
a(n) _____ absorbs moisture or promotes the retention of moisture.
Answers
A hygroscopic substance absorbs moisture or promotes the retention of moisture.
Hygroscopic substances can be found in various forms, including solids, liquids, or even gases. Common examples of hygroscopic substances include salt, sugar, silica gel, certain types of wood, and many chemicals used in industries or laboratories.
The absorption or retention of moisture by hygroscopic substances can have practical applications. For instance, in food preservation, hygroscopic substances can help maintain the moisture content of food products and prevent them from drying out. In pharmaceuticals, hygroscopic ingredients are used to stabilize and control the moisture content of medications.
Overall, hygroscopic substances play a crucial role in moisture management and preservation across various industries and applications.
To know more about the hygroscopic substance refer here :
https://brainly.com/question/31524625#
#SPJ11
When limestone is decomposed at high temperature and the residue is treated with water, the compound that is formed is
Answers
The compound that is formed is calcium hydroxide.
Preparation of calcium hydroxideWhen limestone is decomposed at a high temperature, calcium oxide is formed as the residue.
\(CaCO_3 --- > CaO + CO_2\)
When the calcium oxide residue is treated with water, a calcium hydroxide solution is formed.
\(CaO + H_2O -- > Ca(OH)_2\)
More on calcium hydroxide can be found here: https://brainly.com/question/9584549
#SPJ1
Comparing the sizes of these atoms, which atom is smallest?
a.rubidium
b.zirconium
c.lead
d.silver
e.xenon
Answers
Answer:
xenon
Explanation:
Rb>Zr>Ag>Pb>Xe
What is the mass of 10.0 mol CH₂O2?
10.0 mol CH₂O2 46.03 g CH₂O₂
1 mole CH₂O₂
[?] g CH₂O₂
Answers
Answer:
460 g
Explanation:
We have been asked to determine the mass of 10 mol CH2O2.
We know that 1 mol CH2O2 = 46g.
Thus to find 10 mol CH2O2 we cross multiply as shown below;
mass = (10 mol x 46 g)/(1 mol)
= 460 g of CH2O2
Therefore the mass of 10 mol CH2O2 is 460 g
When mixing 5.0 moles of HZ acid with water up to complete a volume of 10.0 L, it is found that at
reach equilibrium, 8.7% of the acid has become hydronium. Calculate Ka for HZ. (Note: Do not assume is disposable. )a. 1.7×10^−3
b. 9.5×10^−2
C. 2.0×10^−2
d. 4.1×10^−3
e. 3.8×10^−3
f. 5.0×10^−1
Answers
therefore the correct option is d) 4.1×10⁻³.
Given that the initial concentration of HZ is 5.0 moles and at equilibrium, 8.7% of the acid has become hydronium.
The concentration of HZ that has not reacted is (100% - 8.7%) = 91.3%.
The final concentration of HZ is 5.0 × 0.913 = 4.565 moles.
The final concentration of the hydronium ion is 5.0 × 0.087 = 0.435 M.HZ ⇌ H+ + Z-Ka
= [H+][Z]/[HZ]Ka
= [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041
Which is the same as 4.1 × 10-3.
We know that HZ is an acid that will partially ionize in water to give H+ and Z-.
The chemical equation for this reaction can be written as HZ ⇌ H+ + Z-.
The acid dissociation constant (Ka) of HZ is the equilibrium constant for the reaction in which HZ ionizes to form H+ and Z-.Thus, Ka = [H+][Z]/[HZ].
The given problem is a typical example of the dissociation of a weak acid in water. We are given the initial concentration of HZ and the concentration of hydronium ions at equilibrium.
To find the equilibrium concentration of HZ, we can use the fact that the total amount of acid is conserved.
At equilibrium, 8.7% of HZ has dissociated to give hydronium ions.
This means that 91.3% of the original HZ remains unreacted.
Therefore, the concentration of HZ at equilibrium is 5.0 × 0.913 = 4.565 M.
The concentration of hydronium ions at equilibrium is 5.0 × 0.087 = 0.435 M.
Using the equation Ka = [H+][Z]/[HZ], we can substitute the values of the concentrations and the equilibrium constant into the equation and solve for Ka.
Ka = [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041 or 4.1 × 10-3.
The answer is d) 4.1 × 10-3.
To know more about hydronium visit:
https://brainly.com/question/14619642
#SPJ11
a certain element x is made up of two isotopes, one with an atomic mass of 63.9358 that is 48.632% abundant, and one with an atomic mass of 65.9343 that is 51.368% abundant. calculate (to the correct number of significant figures) the average atomic mass for x.
Answers
32.0662
We have given an element X which is made up of two isotopes, one isotope has an atomic mass of 63.9358, which is 48.632% abundant, and the other isotope has an atomic mass of 65.9343, which is 51.368% abundant. We need to calculate the average atomic mass for X. Solution: We know that: Atomic mass = (mass of isotopes 1 x % abundance of isotope 1) + (mass of isotope 2 x % abundance of isotope 2)/100. Let the atomic mass of element X be m. Then, Substituting the values of the atomic mass and abundances, we get, m = (63.9358 × 48.632) + (65.9343 × 51.368)/100= 31.045288 + 33.991912/100= 32.0662. Average atomic mass for X is 32.0662 rounded to 5 significant figures.
Learn more about atomic mass
brainly.com/question/25774151
#SPJ11
Write the balanced equation for the reaction of dilute sulfuric acid. H2so4 , with sodium hydroxide
Answers
Answer:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Explanation:
This question is describing the neutralization reaction between dilute sulfuric acid (H2SO4) and sodium hydroxide (NaOH). These two compounds combine to form sodium salt and water as products (see the chemical equation below).
H2SO4 + NaOH → Na2SO4 + H2O
However, this equation is not BALANCED because the number of atoms of each element are not the same on both sides of the equation. To balance the equation, COEFFICIENTS are used as follows:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
From this equation, the atoms of hydrogen (H), sulphur (S), oxygen (O), sodium (Na) are now equal on both sides, hence, it is said to be BALANCED.
We are studying the ideal gas law. In this discussion, you will be trying your hand at applying one of the ideal gas laws to a real world situation. Consider a situation that involves an ideal gas law and discuss how you would apply your chosen ideal gas law to the situation. Generate an ideal gas law question based on this situation.
Please do not forget to generate a question.
Answers
The ideal gas law, which relates the pressure, volume, temperature, and number of moles of an ideal gas, can be applied to real-world situations. By considering a specific scenario and applying the ideal gas law, we can analyze the behavior of gases and make predictions about their properties.
Let's consider a situation where a scuba diver is exploring underwater at a depth of 30 meters. We can apply the ideal gas law, specifically the form known as Boyle's law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Question: How does the pressure of the gas in the scuba tank change as the diver descends to a depth of 30 meters, assuming the temperature remains constant?
To answer this question, we can use the ideal gas law equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. By keeping the temperature constant, we can observe the relationship between pressure and volume as the diver descends and calculate the change in pressure based on the change in volume.
To learn more about Boyle's law click here:
brainly.com/question/30367133
#SPJ11
What happens if you overheat HNO₃?
Answers
HNO₃ decomposes on heating according to the equation:
4 HNO₃ ---> 2 H₂O + 4 NO₂ + O₂
What is HNO₃?HNO₃ is known as trioxonitrate (v) acid.
It is a volatile acid.
Like other acids, it turns blue litmus solution red and reacts with bases to form salt and water.
Being a volatile acid, when HNO₃ is overheated, it decomposes into several compound.
The equation for the reaction is given below:
4 HNO₃ ---> 2 H₂O + 4 NO₂ + O₂
In conclusion, HNO₃ decomposes on heating.
Learn more about trioxonitrate (v) acid at: https://brainly.com/question/22060503
#SPJ1
anaerobic decomposition of organic waste in rice paddies generates large amounts of
Answers
Anaerobic decomposition of organic waste in rice paddies generates large amounts of methane gas (CH4).
Anaerobic decomposition refers to the breakdown of organic matter in the absence of oxygen. In the case of rice paddies, when organic waste such as plant residues and other organic materials decompose, they undergo anaerobic fermentation. This fermentation process is carried out by microorganisms, particularly bacteria, in the waterlogged conditions of the paddy fields.
During anaerobic decomposition, one of the major byproducts generated is methane gas (CH4). The production of methane in rice paddies is primarily attributed to the activity of methanogenic bacteria that thrive in the oxygen-depleted environment.
The methane produced in rice paddies can be released into the atmosphere through various pathways. It can escape through the water surface, known as ebullition, or be transported through the rice plant tissues and emitted through the leaves, known as plant-mediated transport. Additionally, some methane can be dissolved in the water and later released.
The large amounts of methane generated from the anaerobic decomposition of organic waste in rice paddies contribute to greenhouse gas emissions, which can contribute to climate change and global warming.
To learn more about methane gas (CH4)., refer below:
https://brainly.com/question/31333451
#SPJ11
There is usually only one correct method that can be used to solve a problem true or false
Answers
help in this please....................

Answers
c) because rays contain electrons
which of the following statements describe features found in all elimination reactions?
Answers
In all elimination reactions, **bimolecular elimination** and **formation of a new π bond** are common features.
Elimination reactions are characterized by the removal of atoms or groups from a molecule, resulting in the formation of a new π bond. Two primary types of elimination reactions exist: E1 and E2. In E1 reactions, the leaving group departs first, forming a carbocation intermediate, while in E2 reactions, the leaving group and the proton are removed simultaneously in a concerted process. Both types involve **bimolecular elimination**, where two molecules participate in the reaction. The formation of a new π bond, such as a double bond between carbon atoms, is another feature found in all elimination reactions. These reactions are important in organic chemistry, as they allow for the synthesis of alkenes and other unsaturated compounds.
Know more about elimination reaction here:
https://brainly.com/question/14693649
#SPJ11
PLEASE HURRY WILL GIVE BRAINLIEST
Click Oil Basics and use the information to complete this passage that discusses how plastic production affects society.
The production of plastics makes life
{answer} , so it benefits society. On the other hand, the production of plastics causes air and water {answer} , so it also harms society.
Answers
Answer:
easier and pollution
Explanation:
I just had this question and got it right
A newspaper article about the danger of global warming from the accumulation of greenhouse gases such as carbon dioxide states that "reducing driving your car by 20 miles a week would prevent release of over 1000 pounds of CO2 per year into the atmosphere." Is this a reasonable statement? Assume that gasoline is octane (molecular formula is C8H18) and that it is burned completely to CO2 and H2O in the engine of your car. Facts (or reasonable guesses) about your car's gas mileage, the density of octane, and other factors will also be needed.
Answers
Answer:
The newspaper article's statement is correct since the mass of CO₂ reduced = 1071.85 pounds
Explanation:
Equation for the combustion of gasoline (octane) is given below:
2C₈H₁₈ + 25O₂ ----> 16CO₂ + 18H₂O
Density of C₈H₁₈ = 0.8 g/mL
Assuming mileage of the car is 20 miles/gallon; 1 gallon = 3.785 L
Since 20 miles is reduced in driving each week, thereby saving 1 gallon of gasoline, number of litres of gasoline reduced in a year is given by;
52 * 3.785 L = 196.82 L = 196820 mL
density = mass/volume; mass = density * volume
mass of gasoline saved = 0.8 g/mL * 196820 mL = 157456 g
number of moles = mass/molar mass
Molar mass of C₈H₁₈ = 114 g/mol
number of moles of C₈H₁₈ in 157456 g = 157456 g / 114 g/mol = 1381.2 moles
From the equation of reaction, 2 moles of C₈H₁₈ produces 16 moles of CO₂
1381.2 moles of C₈H₁₈ will produce 1381.2 * 16/2 moles of CO₂ = 11049.6 moles of CO₂
mass = number of moles * molar mass
molar mass of CO₂ = 44 g/mol
mass of CO₂ = 11049.6 moles * 44 g/mol = 486182.4 g
1 g = 0.00220462 pounds
Therefore mass of CO₂ reduced = 486182.4 g * 0.00220462 lb/g
mass of CO₂ reduced = 1071.85 pounds
Therefore, the newspaper article's statement is correct
The gas formed when coal is heated in the absence of air_____________
Answers
Which of the following steps correctly converts 1.25 moles of fluorine to an equivalent mass of fluorine in grams? (5 points)
Add 1.25 to the atomic mass of fluorine.
Divide the atomic mass of fluorine by 1.25.
Subtract 1.25 from the atomic mass of fluorine.
Multiply the atomic mass of fluorine by 1.25.
Answers
Explanation:
Given the amount of fluorine is ---- 1.25 mol.
What is the mass of given fluorine in grams?
Since
\(Number of moles =\frac{given mass of the substance}{its molecular mass}\)
To get the mass of the substance in grams, multiply the given number of moles with the molecular mass of the substance.
Hence, among the given options, the correct answer is the last option that is
Multiply the atomic mass of fluorine by 1.25.
Answer:
Multiply the atomic mass of fluorine by 1.25.
Explanation:
i got it right on the exam!! :)
pls answer question will mark brainliset tyty

Answers
Answer:
Graph D.
Explanation:
A student was given a sample of food and asked to determine the types of nutrients present in the sample. The student placed half of the sample in a test tube with Benedict’s solution and heated it. The solution turned brick red. When an iodine solution was added to the remaining half of the sample, it turned blue black. The student can correctly conclude that the food sample contained
Answers
The food sample contained starch and reducing sugar (carbohydrates).
The Benedict's test is used to test for the presence of reducing sugars, such as glucose, in a sample. When the Benedict's solution is added to a sample containing reducing sugars and heated, the solution will turn brick red.
The iodine test is used to test for the presence of starch in a sample. When iodine solution is added to a sample containing starch, it will turn blue-black.
So, in this case, the student can conclude that the food sample contained both starch and reducing sugars, as both tests produced positive results.
Learn more about Benedict's test and Iodine test here: https://brainly.com/question/25800056
#SPJ4
What is the product of the unbalanced combustion reaction below?
CH₂(g) + O₂(g) →
A. C(s) + H₂(g) + O₂(g)
B. CH₂0(s)
C. C(s) + H₂O(g)
D. CO₂(g) + H₂O(g)
Answers
Explanation:
A is the answers for the question
The product formed in the combustion reaction is generally the oxidized fuel. The combustion reactions are exothermic. The products of the given reaction are CO₂ and H₂O. The correct option is D.
What is combustion?A chemical reaction in which the fuel undergoes oxidation by reacting with a powerful oxidizing agent which results in the release of energy generally in the form of heat is defined as the combustion. All combustion reaction will not results in fires.
The combustion of LPG fuel which is used for cooking food involves the combustion reaction between the oxygen present in the atmosphere and the liquified petroleum gas. The explosion of fire works also indicates the combustion reaction.
The given reaction is given as:
CH₂(g) + O₂(g) → CO₂(g) + H₂O(g)
The substances on the right-hand side are called the products.
Thus the correct option is D.
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ5
Which of the following describes the net reaction that occurs
in the cell,
Cd Cd?*(1 MI Cu?* (1 M) Cu?
a. Cu + Cd?+ - Cu?+ + Cd
b. Cu + Cd - Cu?+ + Ca?+ c. Cu?* + Cd?* - Cu + Cd d. Cu?* + Cd - Cu + Cd?*
e. 2Cu+ Cd?+ > 2Cu* + Cd
Answers
The correct answer is e. The net reaction that occurs in the cell involves the oxidation of copper (Cu) to form copper ions (Cu+), and the reduction of cadmium ions (Cd2+) to form cadmium metal (Cd). This is represented by the equation: 2Cu+ Cd2+ > 2Cu* + Cd.
In this reaction, Cu+ is the oxidizing agent, as it gains electrons and becomes reduced, while Cd2+ is the reducing agent, as it loses electrons and becomes oxidized. This reaction can be used to generate electrical energy in a cell, such as a battery. Overall, the net reaction involves the transfer of electrons from one species to another, resulting in the formation of a metal and an ion.
To know more about Reaction visit:
https://brainly.com/question/30344509
#SPJ11
a student dissolved 1.12 grams of an unknown compound in water in a 250.0 mL volumetric flask and adds water up to the mark. She finds that the concentration of the solution is 0.077 M. what is the molar mass of the compound?
Answers
The molar mass of the unknown compound is 58.2 g/mol.
To find the molar mass of the unknown compound, we need to use the formula:
Molar mass = (mass of compound / moles of compound)
First, we need to calculate the number of moles of the compound in the solution. We can do this by using the formula:
moles = concentration (M) x volume (L)
We are given that the concentration of the solution is 0.077 M and the volume is 0.25 L (since it was made in a 250.0 mL volumetric flask). Plugging these values into the formula, we get:
moles = 0.077 M x 0.25 L = 0.01925 moles
Now, we can use the formula for molar mass to calculate the mass of the compound. We are given that the mass of the compound is 1.12 grams, so:
Molar mass = 1.12 g / 0.01925 moles = 58.2 g/mol
The given problem is about finding the molar mass of an unknown compound that has been dissolved in water. We know the mass of the compound that was added to the solution, as well as the concentration of the resulting solution. Using this information, we can calculate the number of moles of the compound in the solution, which can then be used to find the molar mass.
To calculate the number of moles of the compound, we used the formula: moles = concentration (M) x volume (L). This formula relates the concentration of a solution to the number of moles of solute present in a given volume of solution. By multiplying the concentration (given as 0.077 M) by the volume (0.25 L), we found that there were 0.01925 moles of the compound in the solution.
Once we knew the number of moles of the compound, we could use the formula for molar mass to find the mass of one mole of the compound. This formula is: molar mass = mass of compound / moles of compound. We were given the mass of the compound as 1.12 grams, so we divided this by the number of moles (0.01925) to get a molar mass of 58.2 g/mol.
In conclusion, by using the concentration of the solution and the mass of the compound added to it, we were able to calculate the molar mass of the unknown compound. This information can be useful in identifying the compound and understanding its properties.
To know more about compound visit:
https://brainly.com/question/13516179
#SPJ11
Calculate the amount (mL) of Compound A needed to give 12 mmol. MW of Compound A: 32.04 g/mol Density of Compound A: 0.79 g/mL [x1] mL of Compound A equals 12 mmol (HINT: remember significant digits)
Answers
we need 487.09 mL of Compound A to obtain 12 mmol,
Determine the mass of 12 mmol of Compound A using its molecular weight:
mass = 12 mmol x 32.04 g/mol = 384.48 g
Use the density of Compound A to convert the mass to volume:
volume = mass / density = 384.48 g / 0.79 g/mL = 487.09 mL
A compound refers to a substance that is composed of two or more different elements chemically bonded together. The atoms of these elements are held together by chemical bonds such as covalent or ionic bonds, forming a distinct and unique chemical entity. Compounds have properties that are different from the elements they are composed of, and their properties are determined by the types of atoms present, the arrangement of atoms, and the strength and type of bonds between the atoms.
For example, water is a compound made up of two hydrogen atoms and one oxygen atom, bonded together by covalent bonds. The properties of water, such as its boiling and freezing points, its density, and its ability to dissolve other substances, are unique to water and are a result of its chemical composition and structure.
To learn more about Compound visit here:
brainly.com/question/13516179
#SPJ4
Directions: Answer each of the questions below.
1. Explain how andesite forms.
2. Explain the difference between magma and lava and how lava is formed.
3. Identify some special characteristics of pumice.
4. What are cinder cones and how are they formed.
5. Explain how most continental volcanoes are formed.
Answers
1.) Andesite forms from volcanic eruptions.
2.) Magma is underground while lava is on earth Surface.
3.) Pumice is characterized by being a light rock.
4.) Cinder cones are cones formed around a volcanic vent.
5.) Continental volcanoes are formed around the margin of a continent.
What is the formation of andesite?1.) The andesites are known to be formed during a volcanic eruption which takes place upon rapid cooling of magmas usually when it erupts onto the Earth's surface and forms lava flows.
2.) The difference between magma and lava can be seen in their various formation process. Magma are molten rocks when are formed during volcanic eruption but are stored under the earth surface while lava is molten rock that has reached earth surface through the volcanic vents.
3.) Pumice is an unusually light rock due to the many bubbles inside it.
4.) Cinder cones are cones formed around a volcanic vent. The cinder cones are formed after violent eruptions blow lava fragments into the air, which then solidify and fall as cinders around the volcanic vent.
5.) Most continental volcanoes forms along the margin of a continent where oceanic crust subducts beneath continental crust.
Learn more about volcanic eruptions here:
https://brainly.com/question/23898990
#SPJ1
makes a connection with in chemistry
Answers
Knowing how atoms and molecules behave in chemical reactions in chemistry depends on understanding electron transfer processes. The transfer of one or more electrons from one species.
to another occurs in electron transfer reactions, which are closely connected to oxidation-reduction (redox) processes. Atoms' oxidation states change during redox reactions, which are chemical processes. In the process of oxidation, electrons are lost, whereas in the process of reduction, electrons are gained. In a redox reaction, an oxidizing species (the reducing agent) transfers electrons to a species that is being reduced (the oxidizing agent). The term "oxidized" refers to a species that is losing electrons, whereas the term "reduced" refers to a species that is gaining electrons.Several biological activities, including cellular respiration, photosynthesis, and biochemical reactions, depend heavily on electron transfer reactions. They are utilized in a variety of technologies, including electrochemistry, fuel cells, and batteries.
learn more about electrons here:
https://brainly.com/question/1255220
#SPJ4