What are your initial ideas for what we can do to stop what is occurring to planet earth and ensure the survival of humans and other species?
What can humans do to survive as a species if we are not successful in the negative changes occurring to planet earth?
Answers
Answer: Change the way we run our economy.
Explanation: The way that the world, as a whole, runs its government and economy, is destroying the environment. If we can change this, and find another, better, way to run our countries and cities, the environment will get better, and we won't ruin our planet.
Related Questions
Why is blood liquid?
Answers
Answer:
ok here is you answer
Explanation:
Blood is a liquid because it is composed of cells and plasma that are suspended in a liquid state and can easily flow through the circulatory system, delivering oxygen and nutrients to cells and removing waste products.
mark me as brainliesthow long does it take cream cheese to get to room temperature?
Answers
Since cream cheese has such a high fat content, it doesn't take long to come to room temperature if the room is relatively warm. It takes about thirty minutes on the counter to soften significantly, and about an hour to fully come to room temperature again, depending on the temperature outside and in your kitchen
29. what is complementary base pairing? what is the importance of the order in which base pairs are arranged?
Answers
Complementary base pairing refers to the specific hydrogen bonding interactions between nucleotide bases in a DNA or RNA molecule. The order in which base pairs are arranged is crucial for the structure, replication, and transmission of genetic information.
In DNA, the bases adenine (A) always pairs with thymine (T), and guanine (G) always pairs with cytosine (C). In RNA, thymine is replaced with uracil (U). This pairing follows the rule of A-T/U and G-C base pairing. The specific sequence of base pairs forms the genetic code that determines the unique characteristics and functions of an organism. The importance of the order of base pairs lies in the ability to encode and store genetic information. The sequence of base pairs carries the instructions for protein synthesis, gene expression, and inheritance. The order of base pairs in DNA determines the sequence of amino acids in proteins, which are essential for cellular functions and organismal development.
Any alteration or mutation in the order of base pairs can lead to changes in protein structure or function, potentially resulting in genetic disorders or diseases. Thus, maintaining the correct order of base pairs is vital for the accurate transmission and interpretation of genetic information. Overall, the order of base pairs in DNA and RNA molecules is critical for the storage, expression, and transmission of genetic information, shaping the characteristics and functioning of living organisms.
Learn more about genetic code here:
https://brainly.com/question/21346983
#SPJ11
The reaction represented by the balanced chemical equation below has a AH equal to ____ and energy is_ii__ Combustion of Ethane 2 C₂H6(g) + 7 O2(g) →4 CO₂(g) + 6 H₂O(g) Oi= -84.7 kJ, ii= absor
Answers
The AH value for the combustion of ethane, as represented by the given balanced chemical equation, is -1560.6 kJ/mol. The energy is absorbed during this reaction.
In the balanced chemical equation provided, 2 moles of ethane (C₂H6) react with 7 moles of oxygen gas (O2) to produce 4 moles of carbon dioxide (CO₂) and 6 moles of water vapor (H₂O). The AH value represents the change in enthalpy, or heat, during the reaction per mole of reactant.
To determine the AH value, we can look at the coefficients of the reactants and products in the balanced equation. In this case, 2 moles of ethane react to produce 4 moles of carbon dioxide and 6 moles of water vapor. The AH value is calculated per mole of reactant, so we can divide the AH value by the number of moles of ethane (2) to get the AH value per mole of ethane.
The given value of -84.7 kJ is the AH value per mole of ethane, so we need to multiply it by the number of moles of ethane in the balanced equation (2) to get the total AH value for the reaction. Therefore, the AH value is -84.7 kJ/mol × 2 mol = -169.4 kJ.
However, it's important to note that the given AH value (-84.7 kJ) appears to be incorrect in the question. The actual value for the combustion of ethane is around -1560.6 kJ/mol. This value represents the energy released or absorbed during the reaction. In this case, the negative sign indicates that energy is absorbed, meaning the reaction is endothermic.
In summary, the AH value for the combustion of ethane is -1560.6 kJ/mol, and the energy is absorbed during the reaction.
Learn more about ethane
brainly.com/question/30214217
#SPJ11
There are two isotopes of chlorine. The lighter one with a mass number of 35 (Cl- 35) and the heavier Cl - 37. The atomic mass of chlorine is 35.45 u. Given the mass of chlorine isotopes and the atomic mass of chlorine, determine which isotope is more. Justify your answer.
i need help asap, pls respond quick
Answers
Answer:
Cl-35 isotope is more abundant.
Explanation:
How to calculate the abundance of isotopes in a mixture from the mass of isotopes and the average atomic mass of the element?
The atomic mass of an element having two or more naturally occurring isotopes is calculated using the following relation : Average atomic mass = % abundance of isotope A x atomic mass of isotope A + % abundance of isotope B x atomic mass of isotope B.Solution :
Say the % abundance of Cl - 35 is x, i.e, 100 units of Cl contains x units of Cl-35.
Therefore, the % abundance of Cl - 37 is (100 - x).
∴ [35 x + 37 (100-x)] = 35.45 x 100
Simplifying the above equation, we get
-2x + 3700 = 3545
Subtracting 3700 from both sides of the equation, we get
-2x = -155
or, 2x = 155
Dividing both sides of the equation by 2, we get
x = 155 ÷ 2 = 77.5
∴ 100 -x = 22.5
Thus, Cl-35 is more abundant (77.5%) than Cl-37 (22.5%).
To know more about isotopic abundance, visit:
https://brainly.com/question/24873591
Question
What causes a water molecule to be polar?
A. The Oxygen atom is larger and stronger than the Hydrogen atoms so the electrons spend more of their time nearer the hydrogen
B. The Oxygen atom is smaller and weaker than the Hydrogen atoms so the electrons spend more of their time nearer the oxygen
C. The Oxygen atom is smaller and weaker than the Hydrogen atoms so the electrons spend more of their time nearer the hydrogen
D.The Oxygen atom is larger and stronger than the Hydrogen atoms so the electrons spend more of their time nearer the oxygen
Answers
Answer:
The correct option is D
Explanation:
Water molecules are polar because there structures are made up two sides; the positively charged side (the hydrogen side) and the negatively charged side (the oxygen side). The atoms in the water molecule are joined together by covalent bond - meaning there is sharing of electrons between the constituent atoms. The oxygen atom is bigger/larger (in size) and has a higher electronegativity (ability to attract electrons) hence, the electrons tend to spend more time around the oxygen than the hydrogen atom.
Pls help me with this hw will be thankful!!!

Answers
Answer:
Your missing the entire Transitional Metal group as well as all the Lanthanides and Actinides.
Explanation:
The groups are:
Alkali metals.
Alkaline earth metals.
Transitional metals.
Crystallogens.
Pnictogens.
Chalcogens.
Halogens.
Noble gases.
Find the percentage dissociation and hydrogen ion concentration of 0.2 mol/dm^3 of ethanoic acid if the equilibrium constant of acid is 1.85x10^-5 mol/dm^3
Answers
The percentage dissociation is 0.97% and the hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
To find the percentage dissociation and hydrogen ion concentrationwe need to use the equation:
Ka = [H^+][A^-] / [HA]
where Ka is the equilibrium constant, [H^+] is the hydrogen ion concentration, [A^-] is the concentration of the dissociated form of the acid (acetate ion), and [HA] is the concentration of the undissociated form of the acid (ethanoic acid).
We know the concentration of ethanoic acid, [HA] = 0.2 mol/dm^3, and the value of the equilibrium constant, Ka = 1.85 x 10^-5 mol/dm^3.
Using the equation:
Ka = [H^+][A^-] / [HA]
1.85 x 10^-5 = [H^+][A^-] / 0.2
[H^+][A^-] = 1.85 x 10^-5 * 0.2
[H^+][A^-] = 3.7 x 10^-6 mol^2/dm^6
Since the concentration of the acetate ion is equal to the concentration of the hydrogen ion, [A^-] = [H^+].
So,
[H^+]^2 = 3.7 x 10^-6 mol^2/dm^6
[H^+] = sqrt(3.7 x 10^-6) = 1.93 x 10^-3 mol/dm^3
The hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
To find the percentage dissociation, we divide the concentration of the acetate ion by the initial concentration of ethanoic acid and multiply by 100:
Percentage dissociation = ([A^-] / [HA]) * 100
Percentage dissociation = (1.93 x 10^-3 / 0.2) * 100 = 0.97%.
Therefore, The percentage dissociation is 0.97% and the hydrogen ion concentration is 1.93 x 10^-3 mol/dm^3.
Learn more about hydrogen ion concentration here: brainly.com/question/29022526
#SPJ1
what is an important resonance property of peptide bonds? how does this resonance effect the structure of peptide bonds and the ability of that structure to undergo free rotation?
Answers
One of the critical resonance properties of peptide bonds is the double bond character of the C-N bond.
The double bond resonance within the peptide bond creates a planar structure with restricted rotation.
The nitrogen atom in the peptide bond contains a non-bonding pair of electrons, which generates a double bond character to the peptide bond. This characteristic is the primary reason that peptide bonds show restricted free rotation and planarity.
Peptide bonds are critical in the formation of polypeptides and proteins. These bonds, along with other interactions between amino acids, allow for the complex folding of proteins.
This feature is crucial in the formation of polypeptides and the folding of proteins. The double bond character of peptide bonds is what gives them their distinctive structure and reactivity, allowing them to form the basis of polypeptides and proteins.
To learn about peptide bonds here:
https://brainly.com/question/17296362
#SPJ11
methyl acetate has a chemical composition of 48.64% carbon, 8.16% hydrogen, and 43.20% oxygen. what is the empirical formula?
Answers
8.16 g H is produced by 8.16% H, 48.64 g C by 48.64 g C, and 43.20 g O by 43.20% O. If necessary, divide these numbers by integers to produce whole numbers; if one of the numbers is altered, the others must be altered as well.The empirical formula of \(C3H6O2\) is methyl acetate.
The chemical formula of methyl acetate is 48.64% carbon, 8.16% hydrogen, and 43.20% oxygen. The empirical formula is \(CH2O\). \(CH3N\) empirical formula. \(C3H3O\) is the empirical formula. Consider that we have 100 g of the material. We therefore have 29.0 g of oxygen, 5.5 g of hydrogen, and 65.5 g of carbon. \(C3H3O\) is the empirical formula. A chemical having a mass of only 48.64% C and 8.16% H is composed entirely of carbon, hydrogen, and oxygen. The empirical formula for the chemical is what AI Recommended Reaction This substance's empirical formula is \(C2H6O2\).
Learn more about substance here:
https://brainly.com/question/24372098
#SPJ4
Relative rates of diffusions of two gases X and Y
are found to be 3:2. If the density of Y is 27 the density of x is
Answers
The density of gas X, given that gas Y has a density of 27 is 12
How do i determine the density of X?The following data were obtained from the question:
Rate of gas X (Rₓ) = 3Rate of gas Y (Rᵧ) = 2Density of gas Y (Dᵧ) = 27Density of gas X (Dₓ) =?Graham's law of diffusion states as follow:
Rₓ/Rᵧ = √(Dᵧ/Dₓ)
Inputting the given parameters, we can obtain the density of gas X as shown below:
3 / 2 = √(27 / Dₓ)
Take the square both side
(3 / 2)² = 27 / Dₓ
Cross multiply
(3 / 2)² × Dₓ = 27
Divide both side by (3 / 2)²
Dₓ = 27 / (3 / 2)²
Dₓ = 12
Thus, we can conclude that the density of gas X is 12
Learn more about density:
https://brainly.com/question/28974497
#SPJ1
Select the correct answer. Which chemical reaction shows photosynthesis? A. Water carbon dioxide sunlight → oxygen glucose B. Carbon oxygen → carbon dioxide C. Hydrogen oxygen → water D. Nitrogen hydrogen → ammonia.
Answers
Answer:
C
Explanation:
because they are stressed
Answer:A. Water carbon dioxide sunlight → oxygen glucose
Explanation:
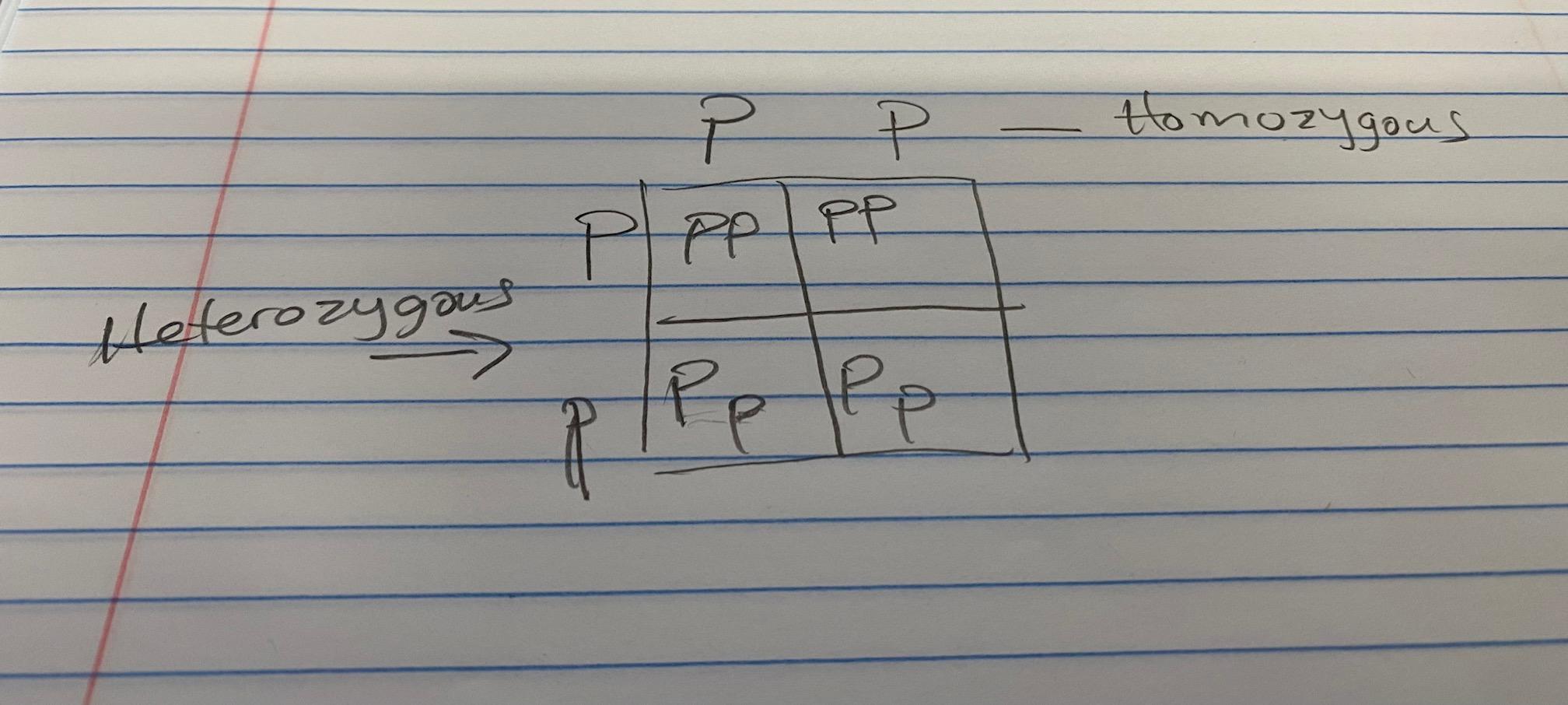
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
the box is like a mini multiplication table
An isotope, cesium-137, has a half-life of 30 years. Starting with 40 grams, how much is left in 60 years?
Answers
You can use a very simple formula for first order decay...
Fraction Remaining (FR) = 0.5n where n is the number of half lives that have elapsed.
In the current problem we want to find the FR, and we know n = 2 half lives elapsed (60 yr/30yr = 2)
FR = 0.52 = 0.25
Since we started with 5.0 g, and we have 0.25 (1/4) left, that would be 5.0 g x 0.25 = 1.25 g
A substance is a solid at room temperature, melts easily, conducts electricity weakly, and dissolves well in water and alcohol. What type of bonds does it have?
Answers
Answer:
Iconic bond
Explanation:
A form of chemical bond that included electrostatic attraction in the oppositely charged ions. It is one of the main types of bonding along with the covalent and metallic bonding. The substance is crystalline in nature since it melts easily and is solid at room temperature.is 2Sb+3L2-> 2SbL3 endothermic or exothermic?
Answers
Answer:
try working out the bond enthalpies for both sides of the equation :)
Explanation:
The heart has four chambers and, within them, blood normally circulates in only one direction: from the right atrium to the right ventricle and from the left atrium to the left ventricle. A certain disease sometimes allows the flow to occur in the opposite direction, that is, it passes from the left ventricle to the left atrium. This disease affects 2% to 3% of the population and rarely produces serious heart problems. Some patients have symptoms such as chest pain, fatigue, palpitation and dizziness. Which heart structure is affected by this disease. How did you reach that conlucsion?
Answers
Answer:
Likely an issue with the mitral valve.
Explanation:
If I read this correctly, you are likely referring to mitral regurgitation, which is a process where your mitral value does not close tightly enough to stop the blood to flow into left atrium.
Normally, when the left ventricle is full, the mitral valve shuts to prevent blood blood from flowing backward into the atrium while the ventricle contracts, and this allows for blood to go to aorta and to body. According to question since the process is reversed, I am gonna go out on a limb and say mitral valve is likely the structure affected by this since and how I came to conclusion is all the explaintion I did earlier.
To increase crop production, food engineers combine two plants so that each
plant's strength compensates for the other's weakness. What is this
technique called?
A. Gene alteration
B. Chemical alteration
C. Crossbreeding
D. Composite materials
Answers
Answer:
C.
Explanation:
Crossbreeding is the correct answer.
Answer:
C. Crossbreeding
Explanation:
In the reaction below green chlorine gas and brown
iodine chloride forms yellow iodine trichloride
Cl2 (g) + ICI () = IC13 (s)
If some chlorine is removed from the reaction, how will
it affect the color of the mixture?
A. It will become more green.
B. It will become more yellow.
C. It will become more brown.
OD. It will not change.
Answers
Answer:
The correct option is;
C. It will become more brown
Explanation:
Given that the reaction is as follows;
Cl₂(g) + ICl (g) ⇄ ICl₃
We have that the reaction and a decrease in the concentration of the reactants will favor the reverse reaction that is the decomposition of the yellow iodine trichloride and the formation of green chlorine gas and brown iodine chloride
Given that some green chlorine gas, which is part of the reactant, will be removed, the reverse reaction will be favored and initial concentration of the yellow iodine trichloride and the green chlorine gas will be reduced while the proportional concentration of the brown iodine chloride will increase and the mixture will become more brown.
Answer:
become more green
Explanation:
how do you balance sulfur + fluoride = sulfur hexafluoride?
S + F2 >>> SF6
Answers
______________
Light energy constitutes minute packets discrete from one another called?
Answers
Light energy constitutes minute packets discrete from one another called as photons.
The Light energy is the electromagnetic radiation. It will exists in the discrete packets of the energy called as the quanta, or the photons. The Photon cannot be subdivided, and the consequently light will always consists of the integer number of the photon. The photon will differ from the each other in the amount of the energy they contain.
The expression for the energy of the photon is as :
E = hv
Where
v is the frequency of light incident.
h is the Planck's constant
Thus , photons is light energy that constitutes minute packets discrete from one another.
To learn more about photons here
https://brainly.com/question/14758126
#SPJ4
long-term potentiation may be involved in long-term memory. choose all of the following that are molecular changes that occur in long-term potentiation. multiple select question. a decrease in the number of nmda receptors entrance of calcium into the dendrite binding of glutamate to nmda receptors the release of nitric oxide which triggers more glutamate release
Answers
The correct ones are:
the release of nitric oxide which triggers more glutamate releasebinding of glutamate to NMDA receptorsentrance of calcium into the dendriteLong-term potentiation (LTP) is a process that involves the continuous strengthening of synapses, resulting in a long-term increase in signal transmission between neurons. It is a critical step in the process of synaptic plasticity. LTP recording is widely accepted as a cellular model for memory research.
Simple tasks can be taught to rats and mice. A mouse, for example, will swim around in a pool of murky water until it finds a hidden platform to climb out on. Long-term potentiation (LTP) of synaptic strength between hippocampal neurons is linked to learning and memory, and memory loss is thought to be caused by LTP dysfunction. LTP can be divided into two types: decaying (early-phase) LTP and nondecaying (late-phase) LTP.
To learn more about long-term potentiation, here
https://brainly.com/question/25677408
#SPJ4
please help with this greatly appreciated





Answers
It is true that according to Hubble's law, the farther away a galaxy is, the faster it is moving away from us.
It is true that the formation of a star occurs when nuclear fusion begins to fuse light elements into heavier ones;
The distance to the nearest stars can be determined by parallax, the apparent shift of a start against background stars observed as the earth moves along its orbit. (Option B)
Based on the accompanying H-R Diagram, the type of start that has the greatest temperature is the blue giants (Option C)
Two hydrogen atoms come together in a nuclear fusion reaction to produce Helium Gas. (Option A).
What is Hubble's Law?Hubble's law, which essentially states that the velocity of a galaxy (or, as it is commonly plotted, its redshift) is precisely proportionate to its distance, also reveals crucial information about the condition of the universe. There should be no relationship between distance and velocity if the cosmos is static and unchanging.
Hubble's law is the physical cosmology observation that galaxies move away from Earth at a rate proportionate to their distance. In other words, the farther they are from Earth, the quicker they are travelling away.
Learn more about Hubble Law:
https://brainly.com/question/13705068
#SPJ1
What type of pollution is pictured here?

Answers
Answer:this air polluction here.
Explanation:
air and soil polluction
Which of the following is a testable hypotheses?

Answers
Answer:
The awser is D.
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, What will be the ion? What will be the number of protons and electrons in the ions?
Answers
An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
An atom X has 12 protons and 12 electrons that means X is a neutral atom and the atomic no. is 12 because total no. of protons is equal to atomic number. When it looses the 2 electrons then atom becomes positively charged ion . the charge on the ion is +2.
now, the no. of protons will be = 12
number of electrons will be = 12 - 2 = 10
Thus, An atom X that has 12 protons and 12 electrons, loses 2 electrons to form an ions, the charge of ion will be X⁺² the number of protons 12 and electrons in the ions is 10.
To learn more about Atom here
https://brainly.com/question/1566330
#SPJ1
how many fluid ounces can i take on a plane united
Answers
Liquid or gel food items larger than 3.4 oz are not allowed in carry-on bags and should be placed in your checked bags if possible. TSA officers may instruct travelers to separate items from carry-on bags such as foods, powders, and any materials that can clutter bags and obstruct clear images on the X-ray machine.
Find the thickness of the material:
This latest alien sample is crazy thin and very tough. After it was cut into something very rectangle-ish with sides of 54.2 cm and 12.3 cm. The calculated density of the material is 1.3 g/cm^3. The mass was found to be 11.4 grams.
Answers
The calculated density of the material is 1.3 g/cm³. The mass was found to be 11.4 grams. The thickness of the material is 14.82 cm³.
What is thickness?Thickness is defined as a dimension, typically the smallest of the three, that describes the separation between two surfaces of an item. There are various layers of fabric that make up a thickness. While density considers how thin or thick strands are collectively, as a group, thickness relates to the breadth of a single hair strand.
Density can be expressed as
Density = mass / volume
Volume = Thickness
So, density = mass / thickness
Thickness = Density x mass
Thickness = 1.3 g/cm³ x 11.4 g
Thickness = 14.82 cm³
Thus, the calculated density of the material is 1.3 g/cm³. The mass was found to be 11.4 grams. The thickness of the material is 14.82 cm³.
To learn more about thickness, refer to the link below:
https://brainly.com/question/11669043
#SPJ1
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
list 3 substances that have atoms which are bonded.
Answers
Answer:
Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur.
(Those are all the ones I know)
Example of 3 substances that have bonded atoms are :
Water ( H₂0 )Calcium Oxide ( CaO )Table salt ( NaCl )Substances that have chemically bonded atoms are generally known as molecules, while a compound is a substance made up by the chemical bonding of different atoms from different elements. there are some readily available substance in nature that have bonded atoms like ; water and Table salt.
Hence we can conclude that three ( 3 ) examples of substances that have bonded atoms are ; Water, Calcium Oxide and Table salt.
Learn more : https://brainly.com/question/21700212
