what determines the types of chemical reactions that an atom participates in? what determines the types of chemical reactions that an atom participates in? the number of protons it contains its atomic number the number of electrons in the outermost electron shell the number of electrons in the innermost electron shell its atomic mass
Answers
The number of electrons in the outermost electron shell determines the types of chemical reactions that an atom participates in.
Related Questions
A sample of neon gas has a volume of 7.2 mL at a pressure of 1.5atm. What is the pressure exerted by the gas if the volume is increased to 28.8 mL at constant tempature
Answers
The pressure exerted by the neon gas, when the volume is increased from 7.2 mL to 28.8 mL at constant temperature, can be calculated using Boyle's Law. The pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
Boyle's Law states that at constant temperature, the product of the pressure and volume of a gas remains constant. Mathematically, it can be expressed as P₁V₁ = P₂V₂. This law allows us to calculate the change in pressure when the volume changes.
In this case, the initial volume (V₁) is given as 7.2 mL, and the initial pressure (P₁) is 1.5 atm. The final volume (V₂) is 28.8 mL. By substituting these values into Boyle's Law equation, we can solve for the final pressure (P₂).
When we perform the calculations, we find that the pressure exerted by the neon gas, when the volume is increased to 28.8 mL, is 0.375 atm. As the volume increases, the pressure decreases due to the inverse relationship between pressure and volume.
Using Boyle's Law: P₁V₁ = P₂V₂
Given:
Initial volume (V₁) = 7.2 mL
Initial pressure (P₁) = 1.5 atm
Final volume (V₂) = 28.8 mL
To find the final pressure (P₂):
P₂ = (P₁ * V₁) / V₂
= (1.5 atm * 7.2 mL) / 28.8 mL
= 0.375 atm
Therefore, the pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
for such more questions on pressure
https://brainly.com/question/24719118
#SPJ8
Brainliest if correct or good answer.
1. What is a GMO, or in other words, Genetically Modified Organism?
Give some reasons why GMO is bad for you.
Answers
Resistance to certain pests, diseases, or environmental variables as well as resistance to chemical treatments are frequently a living thing whose genome contains foreign DNA.
Genetically modified organisms are described as having had their DNA altered via genetic engineering techniques (GMO). Over thousands of years, mankind have modified species using breeding methods. Years of selective breeding have been used to breed dogs, cattle, and even corn to produce particular desirable traits .
Resistance to particular pests, diseases, or environmental elements as well as resistance to chemical treatments are the common objectives of GM crops (e.g. resistance to a herbicide). Another motive for genetic modification, like in the instance of golden rice, is to increase a crop's nutritional value.One method in genetic engineering is the insertion of DNA into the genome of an organism (GE).Learn more about Genome here
https://brainly.com/question/30306185
#SPJ1
a certain reaction has an activation energy of 58.74 kj/mol. at what kelvin temperature will the reaction proceed 7.00 times faster than it did at 337 k?
Answers
A certain reaction has an activation energy of 58.74 kj/mol. At 507 kelvin temperature, the reaction proceed 7.00 times faster than it did at 337 k.
The physical concept of temperature indicates in numerical form how hot or cold something is. A thermometer is used to determine temperature. Thermometers are calibrated using a variety of temperature scales, which historically defined distinct reference points and thermometric substances. The most popular scales are the Kelvin scale (K), which is mostly used for scientific purposes, the Fahrenheit scale (°F), and the Celsius scale, which has the unit symbol °C.
1/T2 - 1/T1 = -Ea/(R×ln(k2/k1))
1/T2 - 1/T1 = -Ea/(R×ln7)
T2 = 1/(1/T1 - Ea/(Rln(7)))
T2 = 1/(1/337 - 58.74/(8.314×1.94591)) = 507 K
To know more about temperature, here:
https://brainly.com/question/7510619
#SPJ12
what is the name of the covalent compound N5Cl8
Answers
Answer:
Did you put that in right because i've never heard of a compound called that nor is there a compound called that on the internet
Explanation:
You must of put that in wrong
example of neutral outcome ?

Answers
Answer:
drops a glass cup of water outcome mad parents
Explanation:
Mad pagents mean death no surviva.
Xenon and fluorine will react to form binary compounds when a mixture of these two gases is heated to 400c in a nickel reaction vessel. at 100.0-ml nickel container is filled with xenon and fluorine, giving partial pressures of 1.24atm and 10.10 atm, respectively, at a temperature of 25c . the reaction vessel is heated to 400c to cause a reaction to occur and then cooled to a temperature at which f2 is a gas and the xenon fluoride compound produced is a non volatile solid. the remaining f2 gas is transferred to another 100.0 -ml nickel container, where the pressure of f2 at 25c is 7.62atm. assuming all of the xenon has reacted, what is the formula of the product?
Answers
assuming all of the xenon has reacted, The formula of the product is XFe2
In this situation, xenon and fluorine are reacting to form a binary compound. The reaction can be viewed by the below balanced chemical equation:
Xe + 2F2 -> XeF2
The reactants, xenon and fluorine, are initially present at partial pressures of 1.24 atm and 10.10 atm, respectively.Since the reactants are initially present at a total partial pressure of 1.24 atm + 10.10 atm = 11.34 atm, and the final partial pressure of fluorine is 7.62 atm, the partial pressure of the xenon fluoride compound must be 11.34 atm - 7.62 atm = 3.72 atm.
we can set up the following equation:1.24 atm + 10.10 atm = 3.72 atm + 7.62 atm
Solving for the number of moles of fluorine gives:
10.10 atm - 3.72 atm = 7.62 atm
= 6.38 atm
Since the pressure of a gas is directly proportional to the number of moles of the gas present,
n = P / (R * T
= 6.38 atm / (0.08206 Latm/molK * 298 K / 100 mL)
= 0.5 molThe number of moles of xenon present can be calculated in a similar way:
n = P / (R * T
= 1.24 atm / (0.08206 Latm/molK * 298 K / 100 mL)
= 0.05 mol
Since the ratio of moles of xenon to moles of fluorine in the product is 1:2, the formula of the product must be XeF2.
To learn more about xenon here:
https://brainly.com/question/5516586
#SPJ4
Easy question
Who is the youngest in ateez
Answers
Answer: jongho
Explanation:
Answer:
Choi JongHo
Explanation:
balanced chemical equation:Copper( ii) oxide solid +dilute sulphuric acid
Answers
Answer:
CuO + H2SO4 = CuSO4 + H2O
Reaction type: double replacement
Staygold1967 avatar
Staygold1967
1 hour ago
Chemistry
High School
PLEASEEE HELPPPP!
1. Your original sample of Manganese-56 is 20.0 mg. How much is left after 3 half
lives? Remember to round to the correct number of significant figures and use units (mg).
2. Tritium is a radioactive form of hydrogen with a half-life of 12.3 years. How much of the 48.0 mg of tritium is still radioactive after 98.4 years?
Make sure to round to the correct number of significant figures and use units (mg).
3. How much of a 1.00 g polonium-214 sample remains after 818 microseconds? The half-life of polonium-214 is 163.7 microseconds.
Make sure to round to the correct number of significant figures and use units (g).
Answers
Given: sulfur, 3.04 g, 1.47 cm³
Wanted: density of sulfur in g/cm³?
Answers
Answer:
2.068
Explanation:
D = M/V
3.04 /1.47
= 2.068
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of aluminum oxide produced by the reaction of 15.0 g of aluminum metal.
[ ]grams Al2O3
4 Al + 3 O2 --> 2 Al2O3
**Your answer should be written as XX.X
Answers
Answer: 28.4 g of aluminum oxide is produced by the reaction of 15.0 g of aluminum metal
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} Al=\frac{15.0g}{27g/mol}=0.556moles\)
The balanced chemical equuation is:
\(4Al+3O_2\rightarrow 2Al_2O_3\)
According to stoichiometry :
4 moles of \(Al\) produce == 2 moles of \(Al_2O_3\)
Thus 0.556 moles of \(Al\) will produce=\(\frac{2}{4}\times 0.556=0.278moles\) of \(Al_2O_3\)
Mass of \(Al_2O_3=moles\times {\text {Molar mass}}=0.278moles\times 102g/mol=28.4g\)
Thus 28.4 g of aluminum oxide is produced by the reaction of 15.0 g of aluminum metal.
How many moles of ammonia are in 2.00 moles of ammonia with solution
Answers
In two moles of NH3, there are 12.04 × 10²³ molecules. Ammonia is a gas that is colorless, extremely unpleasant to breathe, and causes choking.
How much ammonia causes harm to people?Concentrations exceeding 5000 ppm typically result in abrupt respiratory arrest, while concentrations between 2500 and 4500 ppm can be lethal in about 30 minutes. Skin injury can be induced by anhydrous ammonia at concentrations higher than 10,000 parts per million.
How else is ammonia used?80% of the ammonium produced by businesses is employed as fertilizer for agriculture. Ammonia is also used to create polymers, explosives, textiles, pesticides, dyes, and other compounds in addition to its various applications.
6.02×10²³ atoms, molecules
one mole NH₃
= 6.02×10²³ molecules
= 2 × (6.02×10²³ )
= 12.04 × 10²³ molecules.
To know more about ammonia visit:
https://brainly.com/question/1432685
#SPJ1
If 3.0 g of Sr-90 in a rock sample remained in 1999,approximately
40. If 3.0 g of Sr-90 in a rock sample remained in 1999,
how many grams of Sr-90 were
present in the original rock sample in 1943? (1) 9.0 g
(2) 6.0 g (3) 3.0 g (4) 12 g
Answers
In 1933, the initial rock sample contained 11.29 grains of strontium-90.
Detailed explanation:The half-life of strontium is 28.8 years. Therefore,
1999 - 1943 = 56 years.
1.94 half-lives for 56 / 28.8
As a result, for each half-life that passes, the radioisotope's remaining amount will double. time-traveling backward.
The quantity left will therefore increase by 1.94 times when time is advanced by 1.94 half-lives.
Consequently, the amount left over in 1943 is
3.0 × (1.94)² = 11.29 grams
What is the shelf life of strontium?Nuclear fallout contains the radioactive element strontium-90, a byproduct of nuclear reactors. The half-life of it is 28 years.
To know more about radioactive element visit:-
https://brainly.com/question/17551878
#SPJ1
why dont nobody love me ANSWER THAT
Answers
Answer:
god loves you i love u
everybody loves you
a cell is constructed using a silver electrode and a copper electrode in their appropriate solutions. e o ag /ag = 0.80 v and e o cu 2 /cu = 0.36 v. what is e°cell?
Answers
The standard cell potential for this constructed cell is 0.44 V.
To calculate the standard cell potential (E°cell) of this particular cell, we need to use the equation E°cell = E°cathode - E°anode.
In this case, the silver electrode (Ag) is the cathode and the copper electrode (Cu) is the anode. Therefore, E°cathode = E°Ag/Ag = 0.80 V and E°anode = E°\(\frac{Cu}{Cu_{2} }\)+ = 0.36 V.
Substituting these values into the equation, we get E°cell = 0.80 V - 0.36 V = 0.44 V.
Therefore, the standard cell potential for this constructed cell is 0.44 V.
Learn more about cell here:
https://brainly.com/question/27524110
#SPJ11
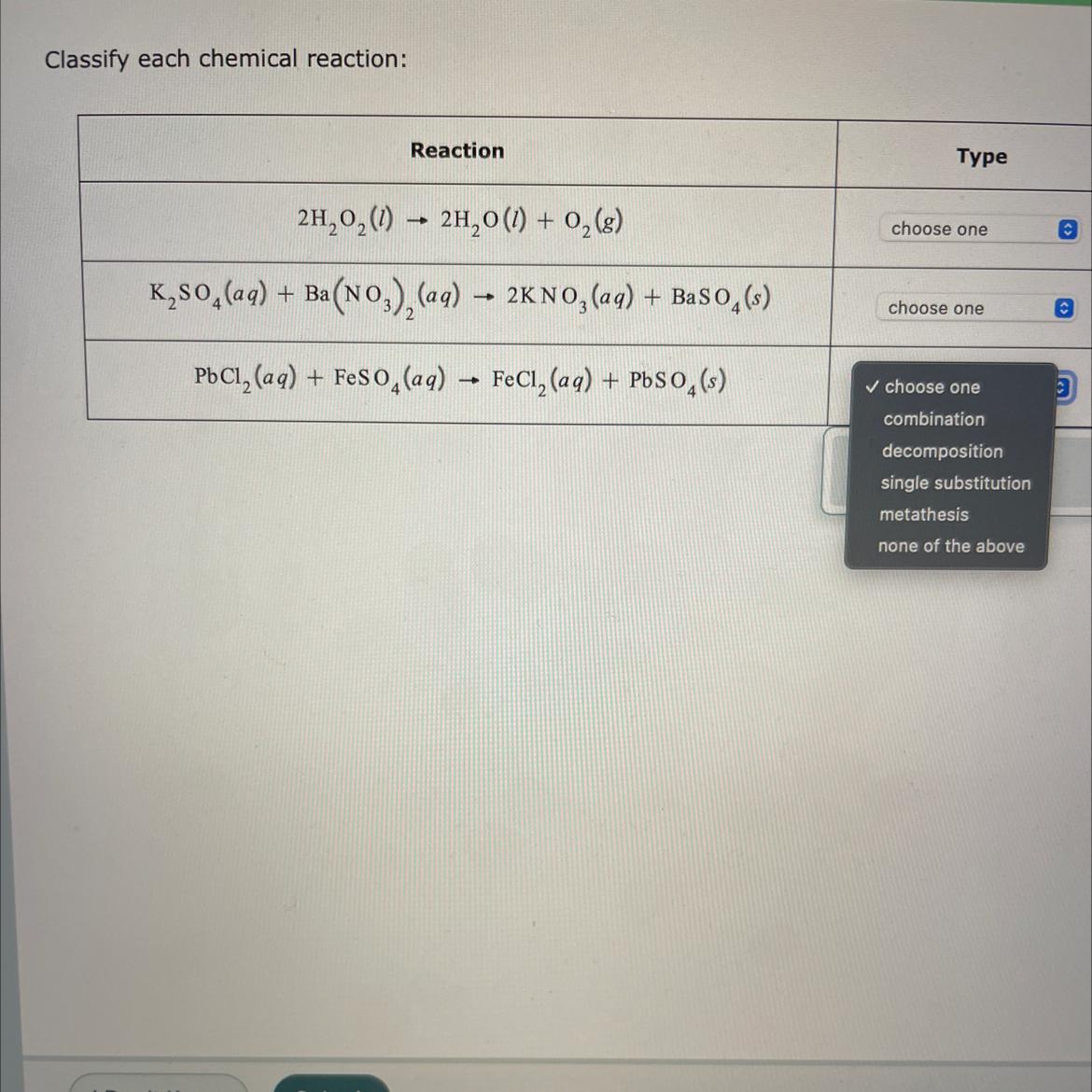
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.
what substances serve many purposes in the purposes in the attempt to move molecules across a plasma membrane
Answers
Carrier proteins serve many purposes in the purposes in the attempt to move molecules across a plasma membrane.
What are carrier proteins?Carrier proteins are proteins which are found n the surfaces of cell membranes which serve the function of transporting molecules across the cell membrane barrier.
The carrier proteins transport various molecules across the cell membrane.
Carrier proteins transport such molecules as sugars, proteins, ions, lipids across the membrane.
Some carrier proteins expend energy in the form of ATP when they transport molecules across the cell membrane.
Some use the concentration gradient of cells to transport molecules across the cell membrane.
The carrier proteins which expend energy to transport molecules are involved in active transport.
The carrier proteins that do not expend energy are involved in passive transport.
In conclusion, carrier proteins are essential in the transport of molecules across the plasma membrane.
Learn more about carrier proteins at: https://brainly.com/question/5556977
#SPJ1
Any shipment of peanuts that contains more than 25 ppb of this dangerous fungus is rejected. A company receives 24 t of peanuts to make peanut butter. What is the maximum mass, in g, of fungus that is allowed? Hint 1 t = 1000 kg.
Answers
Answer:
0.60g of the fungus can be allowed
Explanation:
The maximum concentration of the fungus that can be allowed is 25 ppb, that is 25mg/t.
As the peanuts are 24t, the mass of fungus that can be allowed is:
24t * (25mg/t) = 600mg of the fungus can be allowed. In grams are:
600mg * (1g/1000mg) =
0.60g of the fungus can be allowedAnswer:
0.5g of toxin
Explanation:
ppb = mass of solute/ mass of solution x 10^9
ppb = mass of fungus/ mass of peanuts x 10^9
25ppb = (x/2 x 10^7g) x 10^9
x = (25)(2 x 10^7g)/ 10^9
x = 0.5g of toxin
x represented the unknown mass of solute (fungus), in which we solved for. We already knew we were using the ppb formula, which is mass of solute/ mass of solution x 10^9, because the question mentions that there are 25ppb of peanuts in the shipments. In the calculations, you can see that I substituted the mass of peanuts with 2 x 10^7g. I did this because the question mentions that there are 20t of peanuts and since each t = 1000kg, I multiped 20 x 1000 to get 20 000kg. In my formula for ppb, I know that I have to use grams of solution, not kilograms. Knowing this, we follow the formula for ppb and substitute our known values and solving for unknown values.
Which is the best definition of
force?
A. a push or pull
B. a change in motion
C. a motion that does not change
Answers
Answer:
A
Explanation:
........................
Answer:
push or pull i think..okay?
46 POINTS!!! help me with chemistry, please!
Water Sugar Lemon Juice Lemonade Percent Yield Leftover Ingredients
946.36g 196.86g 193.37g 719.84g 95% Water and Sugar
4. Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. Show your stoichiometric calculations below.
5. During factory inspection, Just Lemons, Inc. discovered that a water valve to the lemonade mixing station was not functioning. Once they repair it, more water will enter the mixing station. From what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers
Answer:
are you sure it is 95%?????
Explanation:
which of these was used by Alfred Wagner as evidence that was continents lead masses have drifted apart over time
Answers
Answer: —The processes of seafloor spreading, rift valley formation, and subduction (where heavier tectonic plates sink beneath lighter ones) were not well-established until the 1960s. These processes were the main geologic forces behind what Wegener recognized as continental drift.
Explanation:
Sodium metal is reacted with chlorine gas to produce 5.85g of a salt.
a. Find the mass of the reacted metal
b. Fid the volume of the reacted gas at STP.
(Na=23, C1=35.5, at STP: 1mole of gas has 22.4L
Answers
Answer:
35.5 this is the answer ok
Answer:
A= 2.3g
B= 1.2L
Explanation:
a) Find the mass of the reacted metal
2Na + Cl2 → 2NaCl
46 71 117
X 5.85
From the chemical equation
117/5.85 = 46/x
X= (46x5.85)/117
= 2.3g
So, the mass of the reacted metal is 2.3g
b) Find the volume of the reacted gas at STP
at STP: 71g of CL2 has 22.4l
Y of Cl2 has V
V= (Y x 22.4L) / 71 where y=?
From the chemical equation
117 / 5.85 = 71 / Y
Y= (71x5.85) / 117
= 3.55
V= (3.55x22.4) / 71
= 1.12L
So, the volume of the reacted gas at STP is 1.12L
What is the pH of a solution that has a [H*] of 3.2 x 10-4?
|
Answers
Answer:
3.495
Explanation:
pH = -log of H⁺ concentration, so the answer is 3.495
1. The respiratory system depends on the nervous system for signals to:
Answers
Answer:transfer oxygen through your blood
Explanation:
Answer:
coordinate the muscles that control breathing
hope this helps ⭐
consider two ionic solids, both composed of singly-charged ions, that have different lattice energies. which solid will be more soluble in water, the one with the larger lattice energy or the one with the smaller lattice energy? assume that solute-solvent interactions are the same for both solids.
Answers
The solid with the smaller lattice energy will be more soluble in water.
This is because the lattice energy represents the energy required to break apart the ionic solid and separate the ions. Therefore, the larger the lattice energy, the stronger the bonds between the ions and the more difficult it is for water molecules to break them apart and dissolve the solid. On the other hand, the smaller lattice energy means weaker bonds between the ions, making it easier for water molecules to interact with and dissolve the solid. So, solubility is inversely proportional to lattice energy.
More on lattice energy: https://brainly.com/question/29735933
#SPJ11
7. The equation for this reaction is shown below.
4CuO(s) + CH4(g) → 4Cu(s) + 2H2O(g) + CO2(g)
-
The water and carbon dioxide produced escapes from the test tube.
Use information from the equation to explain why.
Answers
Answer:
because the lighted splint is burnt and the water and carbon dioxide starts to explode
N2 + 3H2 → 2NH3 How many moles of hydrogen are needed to react with 5 moles of nitrogen?
Answers
Answer: 15 mol
Explanation:
From the equation, we know that for every mole of nitrogen consumed, 3 moles of hydrogen are consumed.
So, the answer is 5(3) = 15 mol
Somebody please help me!! What happens when ionic bonds are formed?
Answers
Answer:
The atom that loses the electrons becomes a positively charged ion, while the one that gains them becomes a negatively charged ion
with the balanced equation : 4Fe + 3O2 = 2Fe2O3How many grams of Fe2O3 is produced when you start with 0.89 moles of iron?
Answers
Answer:
72.66g of Fe2O3 are produced.
Explanation:
1st) From the balanced equation we know that 2 moles of Fe2O3 are produced from 4 moles of iron (Fe). With a mathematical rule of three we can calculate the moles of Fe2O3 that will be produced from 0.89 moles of iron:
\(\begin{gathered} 4molesFe-2molesFe_2O_3 \\ 0.89molesFe-x=\frac{0.89molesFe*2molesFe_2O_3}{4molesFe} \\ x=0.445molesFe_2O_3 \end{gathered}\)Now we know that 0.455 moles of Fe2O3 are produced.
2nd) Now we have to convert 0.455 moles of Fe2O3 into grams, by using the molar mass of Fe2O3 (159.7g/mol):
\(0.455moles*\frac{159.7g}{1mole}=72.66g\)So, 72.66g of Fe2O3 are produced.
You read a primary source and a secondary source that discuss the same
experiment. There is a difference in the conclusions made by these two
sources. Which should you trust more, and why?
A. The secondary source, because it is easier to understand
B. The primary source, because it contains more charts
C. The primary source, because it was written by the researcher
D. The secondary source, because it was printed on paper
SUBM
Answers
Answer:
C
Explanation:
I am not 100% sure, but it might be the answer.
That should be right