what does the subscript 2 stand for following the h in h2o?
Answers
The subscript 2 following the H in H2O stands for the number of atoms of hydrogen in the molecule. In H2O, there are 2 atoms of hydrogen (H) and one atom of oxygen (O).
This combination of atoms forms a molecule of water, represented by the formula H2O. The subscript 2 indicates that there are 2 atoms of hydrogen in this molecule. Water is one of the most abundant molecules on Earth and is essential for all known forms of life. It is composed of two hydrogen atoms bonded to an oxygen atom, forming a covalent bond. Water is a liquid at standard temperature and pressure, and has many unique properties that make it essential for life. Water is a polar molecule, meaning that its positive and negative charges are unevenly distributed.
To learn more about H2O click here https://brainly.com/question/24050121
#SPJ4
Related Questions
Susan conducts an experiment five times and gets a solution concentration of 1.9M, 2.1M, 1.8M, 1.9M, and 2.2M. The known concentration of the solution is 2.0M. Which of the following are true about Susan's results?
A. They are both accurate and precise
B. They are precise but not accurate
C. They are accurate but not precise
D. They are neither accurate nor precise
Answers
What will the pH of 1.50 L of pure water water be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed? What will the pH of the solution in part b be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed?
Answers
Answer:
Part A
pH ≈ 2.273
Part B
ΔpH ≈ -4.726
Part C
pH ≈ 1.973
Part D
ΔpH ≈ -0.301
Explanation:
Part A
The pH of a solution is given by the negative concentration of hydrogen ions in the solution
2.0 mL = 0.002 L
The number of moles of HCl in 2.0 mL of 4.0 M HCl is given as follows;
1 Liter of 4.0 M HCl contains 4.0 moles of HCl
2.0 mL = 0.002 L 4.0 M HCl contains 0.002 L/(1 L) × 4.0 M = 0.008 moles of HCl
The concentration of 0.008 moles in 1.50 L is given as follows;
Concentration = The number of moles/(The volume in liters)
∴ The concentration of 0.008 moles in 1.50 L, C = 0.008 moles/(1.5 L + 0.002 L)
∴ The concentration of 0.008 moles in 1.50 L, C ≈ 0.00533 moles/liter = 0.00533 M HCl
Given that HCl is a strong acid, we have that HCl dissociates completely to give equal number of H⁺ and Cl⁻ ions;
The number of moles of [H⁺] in the solution = 0.00533 moles
The pH of the solution = -log[H⁺]
∴ pH = -log[5.33 × 10⁻³] ≈ 2.273
The pH of the 1.5 L of pure water will be approximately 2.273
Part B
The pH of the pure water has changed from neutral (pH = 7) tp pH = 2.273
The change in pH is ΔpH = 2.274 - 7 = -4.726
ΔpH ≈ -4.726
Part C
When 2.0 mL of the 4.0 M HCl is added, the solution above, we have;
C = (0.008 + 0.008)/(1.5 + 0.002 + 0.002) ≈ 1.06383 × 10⁻²
The concentration of the solution becomes, C ≈ 1.06383 × 10⁻² mole/liter
The pH becomes, pH = -log(1.06383 × 10⁻²) ≈ 1.973
Part D
The amount by which the pH has changed, ΔpH ≈ 1.973 - 2.274 = -0.301.
correct coefficients to balance
Al + ZnO --> Al2O3 + Zn
Answers
Answer:
2 Al + 3 ZnO = 1 Al2O3 + 3 Zn
Explanation:
In order to balance a chemical equation, the atoms on each side of the equation need to be equal. So, now there are 2 Aluminum atoms, 3 Zinc atoms, and 3 Oxygen atoms on each side.How many moles is 118 grams of Argon
Answers
Answer:
\(\boxed {\boxed {\sf About \ 2.96 \ moles \ of \ Argon}}\)
Explanation:
To convert from grams to moles we must use the molar mass, which is found on the Periodic Table.
Argon: 39.9 g/molUse the molar mass as a ratio.
\(\frac{39.9 \ g \ Ar}{1 \ mol \ Ar}\)
Multiply by the given number of grams: 118
\(118 \ g \ Ar *\frac{39.9 \ g \ Ar}{1 \ mol \ Ar}\)
Flip the fraction so the grams of argon will cancel each other out.
\(118 \ g\ Ar * \frac{1 \ mol \ Ar}{39.9 \ g \ Ar}\)
\(118 * \frac{ 1 \ mol \ Ar}{39.9 \ g \ Ar}\)
\(\frac {118 \ mol \ Ar }{ 39.9 }\)
\(2.95739348 \ mol \ Ar\)
The original measurement of grams had 3 significant figures, so our answer must have 3 sig figs.
For the number we calculated, that is the hundredth place. The 7 in the thousandth place tells us to round the 5 to a 6.
\(2.96 \ mol \ Ar\)
There are about 2.96 moles of Argon in 118 grams.
What is the pOH in a solution that has pH = 11.39?
Answers
Answer:
2.61
Explanation:
pH+pOH=14, so sub in 11.39 for pOH, then subtract it over to get pH=2.61
if you have any questions, leave them in the comments and i will try to answer them, if this helped, pls give brainliest.
in the picture on page 2 (it reads page 6 on the bottom of the page) of the bath bombs article, what is the interaction between the water and sodium ions and the water and the bicarbonate ions called?
Answers
The interaction between the water and sodium ions and the water and bicarbonate ions is called a neutralization reaction.
In a chemical reaction, one or more substances (reactants) are changed into one or more new substances (products).
In this case, the reactants are the sodium ions, water, and bicarbonate ions, and the products are sodium bicarbonate, carbon dioxide, and water.
The chemical reaction that occurs when sodium ions and bicarbonate ions combine in water is a neutralization reaction. A neutralization reaction occurs when an acid and a base react together to form a salt and water.
In this reaction, the sodium ions (which act as a base) react with the bicarbonate ions (which act as an acid) to form sodium bicarbonate and water. As a result of this reaction, carbon dioxide is also released.
The reaction can be written as: Na+ + HCO3- → NaHCO3 + H2O + CO2
The interaction between the water and sodium ions and the water and bicarbonate ions is a chemical reaction called a neutralization reaction.
This reaction results in the formation of sodium bicarbonate, water, and carbon dioxide.
to know more about neutralization reaction refer here:
https://brainly.com/question/28970253#
#SPJ11
I need help on this question

Answers
In a metallic bond, electrons _____.
⚪ are shared
⚪ move from a high energy level to a low energy level within one atom
⚪ are completely transferred between bonded atoms
⚪ move freely between the clouds of several atoms
Answers
Answer:
They move freely between the clouds of several atoms, si the correcto Answer is D
Explanation:
Hope this helps.
Who arranged the elements according to atomic mass and used the arrangement to predict the properties of missing element?
Answers
Answer:
Dmitri Mendeleev
Explanation:
Dmitri Mendeleev a Russian Chemist arranged elements on the periodic table according to their atomic mass. He used this arrangement to predict some of the properties of the missing element.
Dmitri Mendeleev around 1869 described the periodic table. The table was based on the periodic law which states that "chemical properties of elements are a periodic function of their atomic weights". In the Mendeleev table, elements are arranged by atomic weights with recurring properties in a periodic manner.What makes up nearly all of the atom's mass?
OA. The sum of all neutrons and electrons
OB. The sum of all protons and electrons
OC. The sum of all isotopes
OD. The sum of all protons and neutrons
SUBMIT

Answers
The total of all protons and neutrons is Option D, which is the right response. Protons, neutrons, and electrons are the three fundamental particles that make up an atom.
The nucleus of an atom is made up of protons and neutrons, which are collectively referred to as nucleons and are primarily responsible for an atom's mass. Neutrons weigh 1.6749 x 10-27 kg, whereas protons weigh 1.6726 x 10-27 kg.
Protons and neutrons make up the majority of an atom's mass when added together. However, electrons contribute very little to the mass of the atom due to their much smaller mass of 9.11 x 10-31 kg. As a result, Option D is the right response.
Learn more about neutrons at:
https://brainly.com/question/28992636
#SPJ1
The mass of an atom may be found by adding the
O protons and neutrons
O molecules
o electrons and protons
O atoms
Answers
Answer:
protons and neutrons
Explanation:
Describe two ways of using solar energy.
Where is the production of hydroelectric energy practical?
Describe two ways to release biomass energy.
Describe two ways to use geothermal energy.
PLEASE ITS DUE IN 6 MINTUES
Answers
Answer:
There are three primary technologies by which solar energy is harnessed: photovoltaics (PV), which directly convert light to electricity; concentrating solar power (CSP), which uses heat from the sun (thermal energy) to drive utility-scale, electric turbines; and solar heating and cooling (SHC) systems, which collect ...
Hydropower is the most important and widely-used renewable source of energy. Hydropower represents about 17% (International Energy Agency) of total electricity production. China is the largest producer of hydroelectricity, followed by Canada, Brazil, and the United States (Source: Energy Information Administration).
There are four ways to release the energy stored in biomass: burning, bacterial decay, fermentation, and conversion to gas/liquid fuel.
Geothermal energy can heat, cool, and generate electricity: Geothermal energy can be used in different ways depending on the resource and technology chosen—heating and cooling buildings through geothermal heat pumps, generating electricity through geothermal power plants, and heating structures through direct-use ...
what is the original source of electrons for psii?
Answers
Answer: In the photosystem II (PSII) reaction center, energy from sunlight is used to extract electrons from water. Explantion: The electron transport chain moves protons across the thylakoid membrane into the lumen.
will magnesium and fluorine atoms most likely form an ionic bond or a covalent bond? 15px
Answers
Magnesium and fluorine atoms will most likely form an ionic bond.
Ionic bonds are formed between elements with a large difference in electronegativity, which is the measure of an atom's ability to attract electrons towards itself. Magnesium and fluorine have a difference in electronegativity of 2.13, which is large enough to form an ionic bond.
In ionic bonds, one atom loses electrons and becomes a positively charged ion (cation), while the other atom gains electrons and becomes a negatively charged ion (anion). In this case, magnesium will lose two electrons to become Mg2+ and fluorine will gain one electron to become F-. These two ions will then attract each other electrostatically to form magnesium fluoride (MgF2), which is an ionic compound.
On the other hand, covalent bonds are formed between elements with a small difference in electronegativity, where atoms share electrons to achieve a stable electron configuration. Magnesium and fluorine have a large electronegativity difference, so they are unlikely to share electrons and form a covalent bond. Therefore, magnesium and fluorine will most likely form an ionic bond.
Learn more about electronegativity here:
https://brainly.com/question/3393418
#SPJ11
which size of micropipette would you select to deliver 215 microliters?
Answers
The size of micropipette that you would select to deliver 215 microliters would be a micropipette with a volume range of 200-1000 microliters.
What is a micropipette?
A micropipette is a laboratory instrument used to measure and dispense small volumes of liquid, typically in the range of microliters (µL) or nanoliters (nL). They are commonly used in chemistry, biology, and biochemistry experiments, as well as in clinical and industrial settings.
Micropipettes consist of a handle, a digital or manual volume adjustment mechanism, and a tip that is placed into the liquid to be dispensed. They work by creating a vacuum or positive pressure inside the tip, which draws or pushes the liquid out of the tip.
Micropipettes come in different volume ranges and it is important to select the right one to ensure accurate delivery of the desired volume. For example, a micropipette with a volume range of 2-10 microliters would not be suitable for delivering 215 microliters, while a micropipette with a volume range of 200-1000 microliters would be more appropriate.
It's also important to note that, even if the micropipette is able to deliver 215 microliters, you should always check the calibration of the micropipette before use, to make sure that it's delivering the correct volume.
Hence, a micropipette with a volume range of 200-1000 microliters is suitable to deliver 215 microliters.
To learn more about a micropipette from the given link:
brainly.com/question/28425080
#SPJ4
Using the data below, what would be the independent variable?
Height of foam
Type of soda
Number of mentos
Color of mentos
Answers
Answer:
Number of mentos
Explanation:
i took the quiz
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
A student in class drops an element on the floor and it shatters quite easily. Which property does this best exemplify?
Answers
Answer:
Glass
Explanation:
1. Necesitas un "cubo concentrado" (de esos que se usan para sazonar las comidas Maggie, ricostilla) dos vasos de vidrio o plástico y agua (caliente y fría). 2.Agrega al vaso con agua fria la mitad de un cubo concentrado y déjala reposar unos minutos. Prepara el segundo vaso con agua caliente y agrégale la otra mitad del cubo. 3. Argumenta tus observaciones en relación con lo observado y con la teoria vista. ¿Como influye la temperatura en este experimento?
Answers
El cubo Maggie es un sazonador que contiene caldo de pollo y grasas, por lo que se disuelve fácilmente al entrar en contacto con el agua caliente gracias a que la temperatura que derrite las grasas y se vuelven líquidas, mientras que con el agua fría no puede disolverse así de rápido ya que las grasas siguen en estado sólido.
Complete and balance the following equation
Pb(OH)2â4(aq)+ClOâ(aq)âPbO2(s)+Clâ(aq) (basic solution)
Answers
The final balanced equation in a basic solution is: \(Pb(OH)_2 + ClO^- + 2OH^- \rightarrow PbO_2 + 2Cl^- + 3H_2O\)
To complete and balance the given equation \(Pb(OH)_2 + ClO^{-} \rightarrow PbO_2 + Cl^-\) in a basic solution, we can follow these steps:
1. Balance the equation for all elements except for oxygen and hydrogen:
\(Pb(OH)_2 + ClO^{-} \rightarrow PbO_2 + Cl^-\)
(The lead and chlorine are balanced in this step)
2. Balance the oxygens by adding water molecules:
\(Pb(OH)_2 + ClO^{-} \rightarrow PbO_2 + Cl^-\) \(+ H_2O\)
(Now, the oxygen atoms are balanced)
3. Balance the hydrogens by adding hydroxide ions (OH−):
\(Pb(OH)_2 + ClO^- + 2OH^- \rightarrow PbO_2 + 2Cl^- + 3H_2O\)
(This step balances the hydrogen atoms)
4. Check if the charges are balanced on both sides of the equation. If not, add electrons (e−) to the side with the more positive charge to equalize the charges.
The charges are already balanced in this equation (+1 on both sides).
The final balanced equation in a basic solution is:
\(Pb(OH)_2 + ClO^- + 2OH^- \rightarrow PbO_2 + 2Cl^- + 3H_2O\)
Learn more about solution :
https://brainly.com/question/30665317
#SPJ11
Which of the chemicals in the following chemical reaction is the acid?
HCO3- + H20 -->CO3^2- +H30+
A.) HCO3-
B.) co3²-
C.) H30+
D.) H20
Answers
A) HCO3- , carbonic acic
PLEASE I NEED HELP ILL GIVE BRAINLYEST
What percentage increase did CO2 experience from 1800 to 2004 AD?
Answers
The percentage of CO2 increased 48 percent. Hope this helped!
can you help me please?

Answers
Explanation:
Physical changes only change the appearance of a substance, not its chemical composition.
Chemical changes cause a substance to change into an entirely substance with a new chemical formula.
Chemical changes are also known as chemical reactions. The “ingredients” of a reaction are called reactants, and the end results are called products
What species are in the buffer region of a weak acid–strong base titration? How are they different from the species at the equivalence point? How are they different from the species in the buffer region of a weak base–strong acid titration?
Answers
The pH will start high and decrease, as opposed to starting low in increasing, but the buffer region is still at the beginning and the equivalence point is still in the middle.
What is weak acid?Corrosive strength is the propensity of a corrosive, represented by the synthetic recipe HA, to separate into a proton, H+, and an anion. Powerless acids don't totally separate into their particles in water. For instance, HF separates into the H + and F - particles in water, yet some HF stays in arrangement, so it's anything but a solid corrosive. There are a lot more powerless acids than solid acids. Most natural acids are feeble acids. A feeble corrosive isn't totally ionized in arrangement. For instance, hydrofluoric corrosive, HF, is a feeble corrosive. At the point when broken up in water, HF particle exist in balance with H +, which responds with water to shape hydronium, and F - particles. Since the corrosive doesn't totally separate into its ionic parts, it is a frail corrosive.
Learn more about weak acid, visit
brainly.com/question/9091286
#SPJ4
Which type of electromagnetic radiation had a lower frequency than infrared radiation
Answers
Answer:
Radio waves
Explanation:
Radio waves, on the other hand, have the lowest energies, longest wavelengths, and lowest frequencies of any type of EM radiation. In order from highest to lowest energy, the sections of the EM spectrum are named: gamma rays, X-rays, ultraviolet radiation, visible light, infrared radiation, and radio waves.
Se disuelven 200 ml de etanol C2H6O al 70 % en volumen con 300 ml de agua calcular considerando que los volúmenes son aditivos El % en volumen de alcohol.
Answers
Answer:
28%
Explanation:
Primero calculamos los mililitros de etanol presentes en la primera solución:
200 mL * 70/100 = 140 mL etanolPara calcular el nuevo porcentaje en volumen, dividimos los mililitros de etanol (que permanecen iguales durante el proceso de dilución) entre el volumen final.
Volumen final = 200 mL + 300 mL = 500 mL% Volumen de Etanol = 140 mL / 500 mL * 100% = 28%What’s the answer to this?
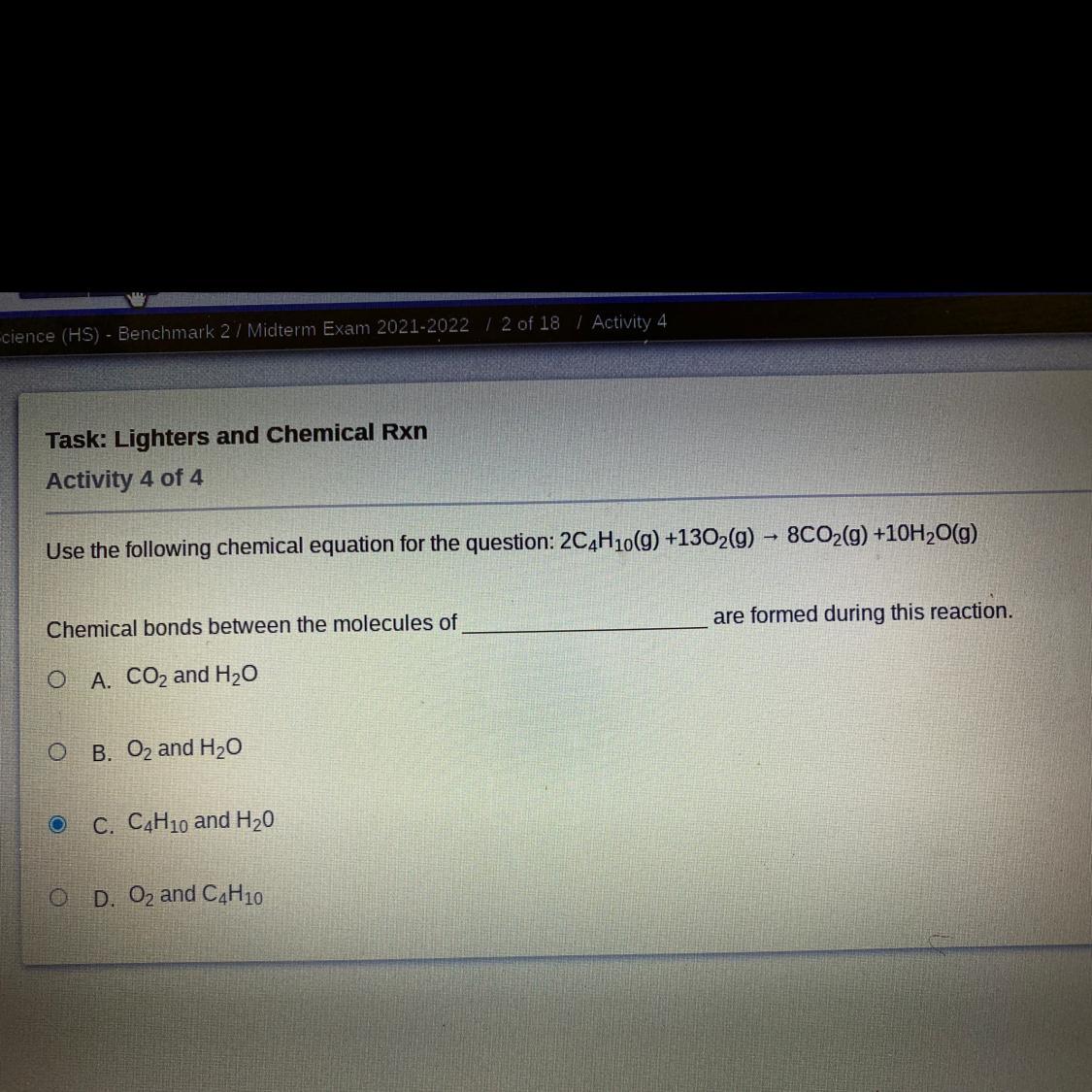
Answers
What is the acronym is a reminder of the most common types of hazards caused by electricity?.
Answers
According to the acronym be safe included in OSHA's Electrocution hazards handbook, an electrical hazard is a situation that occurs at work that exposes employees to the following risks are burns, the most frequent injury brought on by shock.
What is an electrical hazard ?Electric shock and burns from contact with live components are the two primary risks of working with electricity. harm from arcing exposure, fire from improper electrical installations, or both
An electrical danger can be found before it poses a problem by inspecting tools and equipment before use. Regular inspections should be performed on all tools to look for fractures, frayed cord lines, damaged insulation, broken ground pins, and loose parts.
Thus, the Occupational Safety and Health Administration when describing the dangers that electricity may pose to construction workers.
To learn more about electrical hazard, follow the link;
https://brainly.com/question/14374685
#SPJ1
(a) an ideal gas occupies a volume of 1.0 cm3 at 20oc and atmospheric pressure. determine the number of molecules of gas in the container. (b) if the pressure of the 1.0 cm3 volume is reduced to 1.0 x 10-11 pa (an extremely good vacuum) while the temperature remains constant, how many moles of gas remain in the container?
Answers
(a) The number of molecules of gas in the container will be 4.1583 x 10⁻⁵ moles
(b) The moles of gas remaining in the container will be 1.231482x 10⁻²⁰ moles
For a
Volume of gas = 1.0 x 10⁻⁶ m³
Temperature of gas = 20 °C or 293 K
Pressure = 1.013 x 10⁵ Pa
R = 8.3143 J/mol.K
We will calculate the number of molecules from the ideal gas equation.
PV = nRT
Rearrange the equation for the number of moles
n= PV / RT
Put the values
n = (1.013 x 10⁵ Pa) (1.0x10⁻⁶ m³) / ((8.3143 J/mol.K) (293 K))
n = 4.1583 x 10⁻⁵ moles
For b
Volume of gas = 1.0 x 10⁻⁶ m³
Temperature of gas = 20 °C or 293 K
Pressure = 1.0 x 10⁻¹¹ pa
We will calculate the number of moles from the ideal gas equation.
PV = nRT
Rearrange the equation for the number of moles
n = PV / RT
n = (3.0 x 10⁻¹¹ Pa) (1.0 x 10⁻⁶ m³) / (8.3143 J/mol.K) (293 K)
n = 1.231482 x 10⁻²⁰ mol
You can also learn about ideal gas from the following question:
https://brainly.com/question/28257995
#SPJ4
If you had excess chlorine, how many moles of of aluminum chloride could be produced from 25. 0 g of aluminum?Express your answer numerically in moles
Answers
To determine the number of moles of aluminum chloride (AlCl3) that can be produced from 25.0 grams of aluminum, we need to consider the balanced chemical equation and use stoichiometry.
The balanced chemical equation for the reaction between aluminum and chlorine is:
2Al + 3Cl2 → 2AlCl3
From the equation, we can see that the molar ratio between aluminum and aluminum chloride is 2:2 or 1:1. This means that for every mole of aluminum, we can produce one mole of aluminum chloride.
First, we need to calculate the moles of aluminum using its molar mass:
molar mass of aluminum (Al) = 26.98 g/mol
moles of aluminum = mass of aluminum / molar mass of aluminum
moles of aluminum = 25.0 g / 26.98 g/mol
moles of aluminum ≈ 0.926 moles
Since the molar ratio between aluminum and aluminum chloride is 1:1, the number of moles of aluminum chloride produced will be the same as the moles of aluminum:
moles of aluminum chloride = 0.926 moles
Therefore, if there is, approximately 0.926 moles of aluminum chloride can be produced from 25.0 grams of aluminum.
To know more about stoichiometry click this link -
brainly.com/question/28780091
#SPJ11