what element can scratch a diomand?
Answers
Answer:
another diamond ....theres nothing else that can that I know of.
Related Questions
Balance the chemical equation. Based on the equation, how many grams of chlorine are produced when the sodium chloride decomposes and produces 19.5 grams of sodium? Use the periodic table to get the weights of the elements.
Drag the labels to the correct locations to complete the analysis. Each label can be used more than once.
]
Answers
Answer:
Answer is in the photo
Explanation:
Have a nice day and if the photo isn’t visible let me know! :)

The balance chemical equation is as,
\(2NaCl\) → \(2Na + Cl_{2}\)
The 30.1 grams of chlorine are produced when the sodium chloride decomposes and produces 19.5 grams of sodium.
Calculation,
22.99 g of sodium present in 1 mole of sodium atom.
In 19.5 g of sodium = 19.5 g× 1 mole / 22.99 g = 0.85 mole
The mole ratio = 2:1
It means 2 mole of sodium produce along with 1 mole of chlorine when sodium chloride decomposed.
So, number of mole of chlorine when 0.84 mole of sodium produce = 0.85 mole×1 mole/ 2 mole = 0.424 mole
number of mole of chlorine = given mass/molar mass of \(Cl_{2}\) = 0.424
Given mass of \(Cl_{2}\) = molar mass of \(Cl_{2}\)× number of moles of \(Cl_{2}\) = 70.9×0.424 = 30.1 gram
What is balanced chemical equation?The symbolic representation in which number of atoms in reactant side is equal to number of atoms in product side.
learn about balance chemical equation,
https://brainly.com/question/15052184
#SPJ2
what functional group would you expect from reaction of a primary amide with each of the following? if nothing occurs write no reaction. 1) lialh4, 2) h3o
Answers
1) The reaction of a primary amide with LiAlH₄ would result in the reduction of the amide functional group to a primary amine.
2) The reaction of a primary amide with H₃O⁺ would result in the hydrolysis of the amide functional group to form a carboxylic acid and ammonia.
1) LiAlH₄ is a strong reducing agent commonly used for the reduction of carbonyl compounds. In the presence of LiAlH₄, the primary amide undergoes reduction, where the carbonyl group (-C=O) is transformed into a primary amine (-NH₂), resulting in the removal of the oxygen atom.
2) H₃O⁺ represents an acidic environment and can initiate the hydrolysis of amides. In the presence of H₃O⁺, the amide functional group undergoes hydrolysis through a reaction called acid hydrolysis. This process cleaves the amide bond, breaking it into a carboxylic acid and an amine. The amine formed in this case would be ammonia (NH₃).
Overall, the reaction of a primary amide with LiAlH₄ results in the reduction to a primary amine, while the reaction with H₃O⁺ leads to the hydrolysis of the amide, forming a carboxylic acid and ammonia.
learn more about hydrolysis here:
https://brainly.com/question/31827573
#SPJ11
An atom moving at its root mean square velocity at 100. °c has a wavelength of. Which atom is it? assume that the atom is the most abundant isotope of an element.
Answers
The atom that moves at its rms velocity at 100°C with a wavelength of 2.31 * 10 m is : SULPHUR ( s )
Determine the molar mass of the atomTo determine the atom we will have to determine the molar mass of the atom
Applying De Broglie equation
λ = h / mv
Vrms = \(\sqrt{\frac{3RT}{M} }\) ---- ( 1 )
Where : λ = 2.31 * 10⁻¹¹, R = 8.314 J / k.mol, T = 373 K, h = 6.626 * 10⁻³⁴ J.s
From equation ( 1 )
M = ( h² Ua ) / 3RT*λ² --- ( 2 )
where : Ua ( mass of an atom ) = 6.022 * 10²³, h = 6.626 * 10⁻³⁴, R = 8.314 J / k.mol, λ = 2.31 * 10⁻¹¹, T = 373 K
Insert values into equation ( 2 )
M ( molar mass ) = 32 g/mol
Sulphur has a molar mass of 32 g/mol therefore the atom is sulphur.
Hence we can conclude that The atom that moves at its rms velocity at 100°C with a wavelength of 2.31 * 10 m is : SULPHUR ( s ).
Learn more about sulphur : https://brainly.com/question/26328290
what is the purpose of sodium carbonate in part 1a? why do we add glacial acetic acid in part 1b when we react with nn dimethylaniline but we don't use it with the other aromatic coupling reagents
Answers
The Part 1a, the purpose of sodium carbonate is to act as a base and deprotonate the acidic hydrogen present in the compound, which can be a phenol or a carboxylic acid. This deprotonation forms a negatively charged species, called a phenoxide ion or a carboxylate ion.
The more nucleophilic and can undergo the desired reactions more readily, such as electrophilic aromatic substitution. In Part 1b, glacial acetic acid is added when reacting with N, N-dimethylaniline because this compound is a weakly basic amine. The glacial acetic acid serves to protonate the nitrogen atom in the amine, forming an ammonium ion. This step prevents the amine from acting as a nucleophile and reacting with the electrophile that will be used for the aromatic coupling reaction. This ensures that the reaction takes place at the aromatic ring instead of the amine group. For other aromatic coupling reagents that don't have a basic nitrogen atom, there is no need for glacial acetic acid, as they don't require protonation.
learn more about sodium carbonate here.
https://brainly.com/question/31344166
#SPJ11
What is the oxidation number of oxygen in hydrogen peroxide
Answers
Answer:
peroxide such as hydrogen peroxide In peroxide, oxygen has an oxidation number of -1. when oxygen is combined with fluorine it's oxydation number is +2
Explain how the N:Z ratio can help predict the type of radiation Zr-85 and Zr-95 emit
Answers
The N:Z ratio is the ratio of the number of neutrons (N) to the number of protons (Z) in an atom.
What is ratio?
The relation between two numbers which shows how much bigger one quantity is than another.
This ratio can be used to predict the type of radiation emitted by an atom because the stability of an atom is directly related to its N:Z ratio. An atom that has too few or too many neutrons compared to its number of protons is unstable and will attempt to become more stable by releasing radiation.
For example, Zr-85 has an N:Z ratio of 1.18, while Zr-95 has an N:Z ratio of 1.05. This indicates that Zr-85 is more likely to emit beta radiation, while Zr-95 is more likely to emit alpha radiation. Beta radiation is released when an atom has too many neutrons, while alpha radiation is released when an atom has too few neutrons.
To know more about ratio click
https://brainly.com/question/2914376
#SPJ4
how to calculate the mass percent of hydrogen
Answers
Answer:
divide the mass of element in 1 mole of the compound by the compound's molar mass and multiply the answer by 100.
Explanation:
take the molar mass of hydrogen in the water molecule, divide by the total molar mass of water, and multiply by 100.
does it help?
at what points does the object accelerate please explain
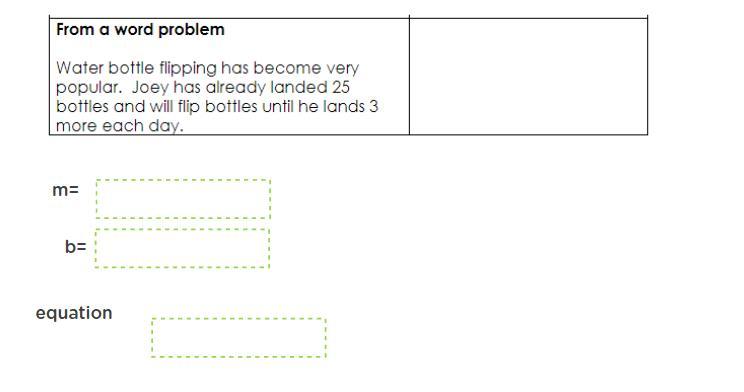
Answers
Answer:
M=3
B=25
Equation= 3x+25
Explanation:
M is your slope and if you follow the slope- intercept formula (y=m+b) you just need to plug in the numbers.
Thus your answer:
M=3
B=25
Equation= 3x+25
Describe the octet rule.
Answers
Answer:
The octet rule is a chemical rule of thumb that reflects the observation that main group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
Explanation:
use the periodic table to select the element that best fits each of the following descriptions. which element below has properties of both metals and nonmetals?
zinc aluminum copper boron
Answers
Answer: Boron is the element which has properties of both metals and nonmetals.
Explanation:
Metals are defined as the elements which loose electrons to attain stable electronic configuration. They attain positive charge and form cation. Example: Zinc (Zn), Aluminium (Al) , copper (Cu)
Non-metals are defined as the elements which gain electrons to attain stable electronic configuration. They attain negative charge and form anion. Example: Chlorine (Cl) , Sulphur (S)
Metalloids are defined as the elements which show properties of both metals and non-metals. There are 7 metalloids in the periodic table. They are Boron (B) , Silicon (Si) , Germanium (Ge) , Arsenic (As) , Antimony (Sb), Tellurium (Te) and Polonium (Po).
Thus boron is the element which has properties of both metals and nonmetals.
Answer:
The answer is A boron!!!!!!
Explanation:
i took the quiz on ed
What is the mass of oxygen in 1 mole of NaHCO3?
Answers
Answer:
48.00
Explanation:
3 oxygen (15.99 x 3)= 48
a block of iron forming a pool of the liquid metal is correctly classified as
Answers
A block of iron forming a pool of liquid metal is correctly classified as a physical change.
This is because although the iron has changed from a solid state to a liquid state, its chemical composition has not been altered. The change is only physical in nature, and can be reversed by allowing the liquid iron to cool and solidify again.
A block of iron forming a pool of liquid metal is correctly classified as undergoing a phase transition, specifically from the solid phase to the liquid phase. This process is known as melting, and it occurs when the iron reaches its melting point (approximately 1538°C or 2800°F).
To know more about iron forming visit:-
https://brainly.com/question/30373249
#SPJ11
Describe the principle. Involved in Gas chromatography.
Answers
What technological procedures and engineering
solutions are available to prevent coastal erosion and land
deterioration in coastalareas?
Answers
To prevent coastal erosion and land deterioration in coastal areas, various technological procedures and engineering solutions can be employed. These include beach nourishment, constructing seawalls and revetments, building breakwaters and groins, implementing dune restoration, establishing offshore reefs, and considering managed retreat.
Beach nourishment involves adding sand to eroded beaches, while seawalls and revetments act as barriers against waves.
Breakwaters and groins disrupt wave energy, dune restoration utilizes vegetation and dunes for protection, offshore reefs dissipate wave energy, and managed retreat involves relocating infrastructure.
These solutions collectively work to safeguard coastal areas, mitigate erosion, and preserve the land from degradation and damage.
To know more about coastal erosion, refer here:
https://brainly.com/question/33096410#
#SPJ11
Element X has 3 naturally occurring isotopes. The first isotope has an atomic mass of 28.0 amu and a natural abundance of 14.0%. The second isotope has an atomic mass of 29.0 amu and a natural abundance of 66.0%. The third isotope has an atomic mass of 27.4 amu and a natural abundance of 20.0%. What is the atomic mass of element X?
a-27.1 amu
b-28.5 amu
c-28.9 amu
d-29.1 amu
Answers
Answer:
Option B. 28.5 amu
Explanation:
The following data were obtained from the question:
Isotope A:
Mass of A = 28.0 amu
Abundance (A%) = 14.0%
Isotope B:
Mass of B = 29.0 amu
Abundance (B%) = 66.0%
Isotope C:
Mass of C = 27.4 amu
Abundance (C%) = 20.0%
Atomic mass of element X =.?
The atomic mass of element X can be obtained by using the following formula:
Atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100] + [(Mass of C × C%)/100]
Atomic mass of Element X = [(28 × 14)/100] + [(29 × 66)/100] + [(27.4 × 20)/100]
= 3.92 + 19.14 + 5.48
= 28.54 ≈ 28.5 amu
Therefore, the atomic mass of Element X is 28.5 amu
Convert 5.43 x 10-6 Mm to cm
dimensional analysis
plz i need help i have more if someone can help me
Answers
Explanation:
1mm = 1/10 cm
5.43 x 10^-6 mm = 5.43 × 10^-7 cm
Analyze How would tides be affected if the
Moon was farther away from Earth?
Answers
Answer: The farther away the moon is from earth the lower the tides would be.
Explanation: Because of the moons close proximity to Earth its gravity pulls up on water which causes the high and low tides, therefore if the moon is farther away its effect would be lessened.
Because of the moons close proximity to Earth its gravity pulls up on water which causes the high and low tides, therefore if the moon is farther away its effect would be lessened.
What is tide?On the planet, tides rise and fall due to the moon's gravitational pull and the Earth's rotating force. The animals that are most impacted by tidal changes in coastal regions have special survival strategies.
A tide is the term used to describe the seawater's alternating approach and retreat along a shoreline. At high tide, the water reaches the shoreline to the greatest extent. When the tide recedes the most, it is at low tide.
The two primary causes of high and low tides are the Earth's rotation and the moon's gravitational pull on the planet. The Moon's pull is greatest on the side of the Earth nearest to it, and as a result, the oceans rise and high tides are produced.
Therefore, Because of the moons close proximity to Earth its gravity pulls up on water which causes the high and low tides, therefore if the moon is farther away its effect would be lessened.
To learn more about tides, refer to the link:
https://brainly.com/question/9493666
#SPJ2
What are the factors affecting the stability of metal complexes
Answers
Answer:
1. Steric Hindrance: The size of ligands, metal ions and the coordination number must be considered while determining stability.
2. Charge: Charge balance is important to maintain stability in metal complexes. The overall charge of the complex should be neutral, and the ligands must balance the charge of the metal ion.
3. Chelate Effect: Metal complexes with chelating ligands generally show higher stability due to the formation of a ring structure that enhances the stability of coordination bonds.
4. Solvent: The nature of the solvent and its polarity affect the stability of metal complexes. An appropriate solvent should be chosen to ensure optimum stability of the complex.
5. Acid-Base Equilibria: The acidity or basicity of the ligands affects metal complex stability.
6. Temperature: The stability of metal complexes is also temperature-sensitive. A change in temperature can alter the stability of metal complexes.
7. Presence of Other Ions: The presence of other ions such as salts, counter-ions, or other metal ions can affect metal complex stability.
8. Steric Crowding: The presence of bulky groups in ligands can hinder coordination, leading to decreased stability.
which one of the following conditions is always true for a titration of a weak acid with a strong base? the equivalence point occurs at a ph equal to 7. if a colored indicator is used, it must change color rapidly in the weak acid's buffer region. a colored indicator with a pka less than 7 should be used. equal volumes of weak acid and strong base are required to reach the equivalence point. the equivalence point occurs at a ph greater than 7.
Answers
The correct option is D, The equivalence point occurs at a pH greater than 7 for a titration of a weak acid with a strong base. This is because the strong base will react with the weak acid to form a salt and water.
Titration is a commonly used analytical technique in chemistry for determining the concentration of an unknown solution by adding a known amount of a standardized solution of known concentration. The process involves slowly adding the standardized solution to the unknown solution until the chemical reaction between the two is complete. The point at which the reaction is complete is known as the equivalence point and can be detected using various indicators that change color or other properties at this point.
The main aim of titration is to accurately measure the concentration of a particular substance in a solution. For example, an acid-base titration can be used to determine the concentration of an acid in a solution by adding a known amount of a strong base until the equivalence point is reached. Similarly, a redox titration can be used to determine the concentration of a reducing or oxidizing agent in a solution.
To know more about Titration refer to-
brainly.com/question/31483031
#SPJ4
Draw the major organic product of the following reaction, and select the mechanism which would dominate (SN1, SN2, E1, or E2).
Answers
SN1 (Substitution Nucleophilic Unimolecular) and SN2 (Substitution Nucleophilic Bimolecular) are mechanisms that involve the substitution of a nucleophile for a leaving group. SN1 reactions proceed through a two-step process with a carbocation intermediate, while SN2 reactions occur in a single step with a concerted attack by the nucleophile.
E1 (Elimination Unimolecular) and E2 (Elimination Bimolecular) are mechanisms involving the removal of a leaving group and the formation of a double bond. E1 reactions proceed via a carbocation intermediate and involve the removal of a proton and a leaving group. E2 reactions occur in a single step with the simultaneous removal of a proton and a leaving group.
The dominance of a particular mechanism depends on factors such as the nature of the reactants, the leaving group, the nucleophile/base, the solvent, and the reaction conditions. Each mechanism has its own set of conditions under which it is more likely to occur.
To know more about SN1 :
brainly.com/question/30766266
#SPJ11
Stoichiometry Review Worksheet
1
Solid tin reacts with diatomic chlorine to produce tin (IV) chloride. If 95 grams of tir
react in an excess of chlorine, what mass of tin (IV) chloride is produced?
Answers
\(\\ \rm\longmapsto Sn+2Cl_2\longrightarrow SnCl_4\)
Moles of Tin:-
95/118=0.8molHere
1 mol of Tin produces 1 mol Tin chlorideSo 0.8 mol will produce 0.8mol Tin chlorideMass of Tin chloride
\(\\ \rm\longmapsto 0.8(189)=151.2g\)
Select the correct answer.
Fraternal twins are formed when sperms fertilize two different eggs. What would you expect about the
twins' DNA and physical appearance?
They have similar DNA and they have identical physical appearances.
They have different DNA but exactly the same physical appearances.
They have similar DNA but environmental factors might cause changes in their physical
appearance.
They have different DNA and different physical appearance
Answers
Answer:
They have different DNA and different physical appearance
Explanation:
Because if the sperms fertilize two different eggs the DNA will be different too and if the sperms are different the physical appearance will be different also
What is the name if this compound? C6H5-C-H=O
Answers
Answer:
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Provide 4 examples of each of the following, what are they used for and their environmental health and safety impacts: - Natural Nanomaterial - Engineered Nano materials - Organic Nano materials - Inorganic Nanomaterials
Answers
Nanomaterials, whether natural, engineered, organic, or inorganic, offer various applications across industries. However, their environmental health and safety impacts need to be carefully evaluated and managed to mitigate any potential risks.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
Natural Nanomaterials:
Examples: Carbon nanotubes (CNTs) derived from natural sources like bamboo or cotton, silver nanoparticles in natural colloids, clay minerals (e.g., montmorillonite), iron oxide nanoparticles found in magnetite.
Uses: Natural nanomaterials have various applications in medicine, electronics, water treatment, energy storage, and environmental remediation.
Environmental health and safety impacts: The environmental impacts of natural nanomaterials can vary depending on their specific properties and applications. Concerns may arise regarding their potential toxicity, persistence in the environment, and possible accumulation in organisms. Proper disposal and regulation of their use are essential to minimize any adverse effects.
Engineered Nanomaterials:
Examples: Gold nanoparticles, quantum dots, titanium dioxide nanoparticles, carbon nanomaterials (e.g., graphene), silica nanoparticles.
Uses: Engineered nanomaterials have widespread applications in electronics, cosmetics, catalysis, energy storage, drug delivery systems, and sensors.
Environmental health and safety impacts: Engineered nanomaterials may pose potential risks to human health and the environment. Their small size and unique properties can lead to increased toxicity, bioaccumulation, and potential ecological disruptions. Safe handling, proper waste management, and risk assessment are necessary to mitigate any adverse effects.
Organic Nanomaterials:
Examples: Nanocellulose, dendrimers, liposomes, organic nanoparticles (e.g., polymeric nanoparticles), nanotubes made of organic polymers.
Uses: Organic nanomaterials find applications in drug delivery, tissue engineering, electronics, flexible displays, sensors, and optoelectronics.
Environmental health and safety impacts: The environmental impact of organic nanomaterials is still under investigation. Depending on their composition and properties, they may exhibit varying levels of biocompatibility and potential toxicity. Assessments of their environmental fate, exposure routes, and potential hazards are crucial for ensuring their safe use and minimizing any adverse effects.
Inorganic Nanomaterials:
Examples: Quantum dots (e.g., cadmium selenide), metal oxide nanoparticles (e.g., titanium dioxide), silver nanoparticles, magnetic nanoparticles (e.g., iron oxide), nanoscale zeolites.
Uses: Inorganic nanomaterials are utilized in electronics, catalysis, solar cells, water treatment, imaging, and antimicrobial applications.
Environmental health and safety impacts: Inorganic nanomaterials may have environmental impacts related to their potential toxicity, persistence, and release into ecosystems. Their interactions with living organisms and ecosystems require careful assessment to ensure their safe use and minimize any negative effects.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
To know more about Nanomaterials, visit
brainly.com/question/29540028
#SPJ11
A gas at STP has a volume of 37.8 L. If the temperature is raised to 295 K and the pressure is changed to 50.0 kPa, what is the new volume of the gas?
Answers
When a gas at a given temperature and pressure is changed, the new volume of the gas can be calculated using the ideal gas law.
What is the new volume of the gas?The ideal gas law states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.Given the temperature and pressure of the gas, we can rearrange the ideal gas law to solve for V: V = nRT / P. In this equation, n and R are constants. Since the new temperature and pressure are given, we can calculate the new volume of the gas:V = nRT / PV = (n)(0.08206 L•atm/mol•K)(295 K) / (50.0 kPa)V = 45.49 L Therefore, the new volume of the gas at 295 K and 50.0 kPa is 45.49 L, which is an increase of 7.7 L from the original volume of 37.8 L at STP. This is due to the fact that when the temperature and pressure of a gas are increased, the volume of the gas increases as well.To learn more about the ideal gas law refer to:
https://brainly.com/question/25290815
#SPJ1
Metal oxides are in which state?
Answers
Answer:
Metal oxides are crystalline solids that contain a metal cation and an oxide anion. They typically react with water to form bases or with acids to form salts.
Explanation:
Hope this helps!
hi people just saying if you think your life is hard just remember other people have it way worse then you
people likeeeeee me so be grateful
Answers
i mean my mom takes all my electronics at night.
Name three functional groups common to natural products that are best handled by steam distillation relative to other separation techniques. What simple chemical reaction do these all have in common which would destroy the intrinsic character of these functional groups?
Answers
Three functional groups commonly handled by steam distillation are alcohols, aldehydes, and ketones. These groups can be destroyed by oxidation, which would alter their intrinsic character and chemical properties.
Three functional groups common to natural products that are often handled by steam distillation are:
1. Alcohols (-OH)
2. Aldehydes (-CHO)
3. Ketones (-C=O)
These functional groups are best handled by steam distillation because they have relatively low boiling points and are volatile. Steam distillation allows for the separation of these compounds from the non-volatile components of a mixture, such as plant material, without subjecting them to harsher conditions that could lead to degradation or chemical changes.
The simple chemical reaction that these functional groups have in common is oxidation. Oxidation reactions involve the loss of electrons or an increase in the oxidation state of an atom, and they can result in the destruction of the intrinsic character of these functional groups. For example, alcohols can be oxidized to form aldehydes and ketones, while aldehydes and ketones can undergo further oxidation to produce carboxylic acids. These transformations can alter the chemical properties and functionality of the natural products, potentially diminishing their natural characteristics.
Learn more about oxidation here
https://brainly.com/question/13182308
#SPJ11
En un experimento hacemos reaccionar a una solución de acido cloridico con el metal cinc. Para ello, hemos usado, 6,5g de cinc y gas hidrogeno. ¿Cual es la masa de gas obtenida? Urgente es para un examen
Answers
Answer:
0.200g de gas son obtenidos
Explanation:
La reacción de ácido clorhídrico, HCl, con Zn es:
2HCl(aq) + Zn(s) → ZnCl₂(s) + H₂(g)
Donde 2 moles de ácido reaccionan con 1 mol de Zn para producir una mol de cloruro de cinc y una mol de hidrógeno (gas)
Asumiendo que el ácido está en exceso, las moles de 6.5g de Zn (Masa molar: 65.38g/mol) son:
6.5g × (1mol / 65.38g) = 0.0994 moles de Zn
Como 1 mol de Zn produce 1 mol de hidrógeno, las moles de hidrógeno son 0.0994 moles de H₂. En gramos (Masa molar H₂ = 2.01g/mol):
0.0994 moles H₂× (2.01g / mol) = 0.200g de gas son obtenidos
Why does methyl orange is changed into red colur after treating it with hcl?