Answers
0.8mole/L is concentration of N2O5.
As the reaction is first order ,
so the order with respect to N2O5 is 1.
general,
Rate of equation is written as
rate = k [N2O5]^1
k is the rate constant = 3 * 10 ^ - 5 * S ^ - 1
rate = 2.4*10^-5 mole/L
N2O5 is concentration term
2.4*10^-5 mole/L = 3 * 10 ^ - 5 * S ^ - 1 [ N2O5]
N2O5 = [0.8 mole/L]
=0.8mole/L
Learn more about N2O5 here:
https://brainly.com/question/19100869
#SPJ9
Related Questions
What stress causes this type of fault to form? O compression O gravity O tension O shearing

Answers
Answer: It’s D
Explanation:
Cuz I said so
Answer: d. Shearing
Explanation:

Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
16. Which of the following is directly responsible for acid rain? A. steam vented from a nuclear power plant B. sulfur dioxide released from a coal-fired power plant. C. mining of coal for a coal-fired power plant D. processing of uranium for a nuclear power plant
Answers
Answer
B. sulfur dioxide released from a coal-fired power plant.
Explanation
Power plants release the majority of sulfur dioxide and much of the nitrogen oxides when they burn fossil fuels, such as coal, to produce electricity. In addition, the exhaust from cars, trucks, and buses releases nitrogen oxides and sulfur dioxide into the air. These pollutants cause acid rain.
Therefore, what is directly responsible for acid rain is:
B. sulfur dioxide released from a coal-fired power plant.
Assuming ideal solution behavior, what is the boiling point of a solution of 115.0 g of nonvolatile sucrose (table sugar), C₁₂H₂₂O₁₁ (342.300 g/mol), in 350.0 g of water (Kb = 0.512 °C m⁻¹; boiling point = 100.0 °C)?
a.)
100.00049 °C
b.)
99.5 °C
c.)
268.2 °C
d.)
100.5 °C
Answers
The boiling point of water is 100.0 °C, the boiling point of the solution will be : 101.49 °C.The correct answer is option (a) 100.00049 °C.
Ideal Solution : An ideal solution is a homogeneous mixture of two or more components that obeys Raoult's law, which states that each component's vapor pressure is proportional to its mole fraction.The boiling point of a solution depends on the solvent's properties and the solute's concentration. It's dependent on the mole fraction of the solvent and solute, as well as the total concentration of the solution. The change in boiling point of a solution is given byΔTb = Kb × m × i, whereKb = ebullioscopic constant, m molarity of the solution, and i = van't Hoff factor.Assuming that the solution's behavior is ideal, we may use the molality of the solution to compute the boiling point elevation of the solution.The molality of the solution is given by the following formula:m = (n₂ / m₂) ÷ (n₁ / m₁), where n is the number of moles, m is the mass, and the subscripts 1 and 2 refer to water and non-volatile solute sucrose, respectively.The molar mass of sucrose (C₁₂H₂₂O₁₁) is342.3 g/mol; therefore, the number of moles of sucrose is115.0 g ÷ 342.3 g/mol = 0.335 mol.m₁ = mass of water = 350.0 g, and m₂ = mass of sucrose = 115.0 g, as given in the problem.Therefore, the molality of the solution is given by:m = (0.335 mol / 0.115 kg) ÷ (1 mol / 1 kg) = 2.91 mol/kg.Substituting these values in the formula for ΔTb, we get:ΔTb = Kb × m = 0.512 °C m⁻¹ × 2.91 mol/kg = 1.49 °C.100.0 °C + 1.49 °C = 101.49 °C.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
Calculate the energy of a photon of radiation with a frequency of 8.5 x 10¹ Hz
Use this calculator to submit your answer in a decimal form.
Type your answer...
Answers
Answer: 0.85 hertz
Explanation: Calculator said so also 0.85 is decimal form for 85
you discover the head of a match contains 3.75 g of sulfur. How many atoms of sulfur does the match contain? (hint: grams > moles > atoms)
Answers
As the question says, we are going to follow the path of the hint, first by finding the number of moles of Sulfur in 3.75 grams, and we can do that by using its molar mass, which is 32g/mol
32g = 1 mol
3.75g = x moles
32x = 3.75
x = 3.75/32
x = 0.12 moles of Sulfur in 3.75 grams
Now we have the number of moles, and to find the number of atoms, we need to use the Avogadro's constant number, which is the number of atoms in a single mol, this value is 6.02*10^23 atoms in 1 mol
1 mol = 6.02*10^23 atoms
0.12 moles = x atoms
x = 7.22*10^22 atoms of Sulfur in 3.75 grams
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
A salt with a high melting point can be obtained from reaction between metal and
acid solution. The metal that used in this reaction is iron and the acid solution is
hydrochloric acid.
a. What is the chemical name of salt above?
Answers
Iron (II) chloride and hydrogen gas
Chemical name:
FeCl2 (aq) + H2 (g)
Calculate the molarity of 33.9 g of MgS in 969 mL of solution.
Answers
Answer:
0.620M
Explanation:
molarity= moles of solute/volume of solution
molar mass of mgs= 24.3+32= 56.3
moles= 33.9/56.3=0.601
molarity= 0.601/0.969=0.602M
please answer holy its been an hour

Answers
Reverse the sign of the second equation, change the sign of the enthalpy and add. Option B
What is the enthalpy?The enthalpy could be obtained by the use of the Hess law of constant heat summation. As such, we have a number steps and it is possible for use to obtain the enthalpy of the final reaction as shown by a series of manipulations.
By inspection, we can see that, if we desire to obtain the enthalpy of the final reaction as shown, then we have to reverse the sign of the second equation, change the sign of the enthalpy and add. Option B
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
What is the maximum mass of aluminum chloride that can be formed when reacting 28.0 g of aluminum with 33.0 g of chlorine?
Answers
Aluminum and chlorine react to generate aluminium chloride, according to a balanced chemical equation: 2Al + 3Cl2 2AlCl3. A quantity of aluminium chloride up to 139.5 g can be produced.
What is the most amount of aluminium chloride that can be created when 27 g of aluminium and 32 g of chlorine are combined?When the limiting reagent is totally transformed into products, the maximum amount of product is produced. Two moles of aluminium chloride are created by a full reaction between three moles of chlorine. Hence, 46.4 g of aluminium chloride is the maximum mass that can be produced.
The amount of aluminium and chlorine in the specified masses can be calculated as follows:
Number of moles of aluminum = 28.0 g / 27 g/mol = 1.04 mol
Number of moles of chlorine = 33.0 g / 35.5 g/mol = 0.93 mol
We may get the theoretical yield of aluminium chloride using the balanced equation: 2Al + 3Cl2 → 2AlCl3
1.04 mol Al × (2 mol AlCl3 / 2 mol Al) × (133.34 g AlCl3 / 1 mol AlCl3) = 139.5 g AlCl3
To know more about aluminium chloride visit:-
https://brainly.com/question/16088530
#SPJ1
Please if you know the answer put it thanks

Answers
The diagram shows a picture of a compound.
What is a compound?A compound is a substance that is made up of two or more different elements that are chemically bonded together in a specific ratio.
This means that the elements are combined in a way that creates a new substance with different physical and chemical properties than the individual elements.
Compounds can be formed through a variety of chemical reactions, such as combining elements through a chemical bond or through a reaction between an acid and a base.
So for the given diagram, we can see that it represents two or more elements chemically combined.
Learn more about a compound here: https://brainly.com/question/29108029
#SPJ1
KF + 02
Balance the equation
Answers
Explanation:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
Answer:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
Help !!A student examines a picture of an important biological molecule in a textbook and writes four statements about the molecule in a chart
1. The molecule is found in the nucleus,
2. The molecule is made up of chromosomes.
3. The molecule carries genetic information.
4. The molecule contains genes.
Which statement needs to be revised to make it true? statement 1 statement 2 statement 3 statement 4
Answers
Answer:
Statement 2 (The molecule is made up of chromosomes)
Explanation:
This question is describing DNA, as the biological molecule. DNA is a type of nucleic acid that functions in the storage of genetic information in the cell. This means that contained in the DNA, are segments that code for proteins called GENES. It is contained in the nucleus of eukaryotic cells where it is wrapped around structures called CHROMOSOMES.
BASED on the four statements given by the student, which happens to be characteristics of this molecule, the statement 2, which states that the molecule is made up of chromosomes needs to be revised to make it true. This is because DNA is not made up of chromosomes but rather DNA is contained in chromosomes.
Based on this passage, the term "mechanical disintegration" means
breaking into small pieces
separation of solid and liquid
evaporation of gases in talus
cultivation of grains
Answers
Mechanical disintegration means breaking into small pieces (option A).
What is mechanical digestion?Digestion is the process occuring in the gastrointestinal tract, by which food is converted into substances that can be utilized by the body.
Digestion can, however, be mechanical/physical or chemical/enzymatical. The mechanical digestion involves the breaking down of food into smaller pieces by teeth.
Therefore, according to this question, there is no passage, however, the meaning of mechanical disintegration can be easily detected in biology.
Learn more about mechanical digestion at: https://brainly.com/question/15457673
#SPJ1
An unknown element (Uk) has three isotopes, as presented in
the table below. One of the percentage values is missing.
After calculating the missing percent abundance (%).
determine the average atomic mass of Uk.
Enter your answer to the hundredths place.
Percent
Abundance
(%)
Uk-66 65.994 58.00
Isotope Mass
Notation (amu)
Uk-69 68.958
Uk-71 70.975
10.00
?
Answers
The average atomic mass of the element, Uk is 67.44 amu.
What is the average atomic mass of the element, Uk?The average atomic mass of the element Uk is determined from the sum of the isotopic masses of the isotopes of the element and their relative abundances.
average atomic mass = sum of (isotopic mass * relative abundance) of the isotopesThe isotopic masses and relative abundance of the isotopes are given below:
Isotope:Uk-66; Isotopic mass = 65.994; relative abundance = 58.00%
Isotope:Uk-69; Isotopic mass = 68.958; relative abundance = ?
Isotope:Uk-71; Isotopic mass = 70.975; relative abundance = 10.00%
Relative abundance of Uk-69 = 100% - (58 + 10)% = 32%
The average atomic mass of Uk = (65.994 * 58.00%) + (68.958 * 32%) + (70.975 * 10.00%)
The average atomic mass of Uk = 67.44 amu
In conclusion, the average atomic mass of an element is obtained from the of the isotopic masses of the isotopes of the element and their relative abundances.
Learn more about average atomic mass at: https://brainly.com/question/23093395
#SPJ1
Determine the enthalpy of reaction for HCl(g) + NaNO₂(s) → HNO₂(l) + NaCl(s) 2NaCl(s) + H₂O(l) → 2HCl(g) + Na₂O(s) ∆H° = -507.1 kJ/mol NO(g) + NO₂(g) + Na₂O(s) → 2NaNO₂(s) ∆H° = -427.0 kJ/mol NO(g) + NO₂(g) → N₂O(g) + O₂(g) ∆H° = -43.01 kJ/mol 2HNO₂(l) → N₂O(g) + O₂(g) + H₂O(l) ∆H° = +34.02 kJ/mol
Answers
the enthalpy of reaction for HCl(g) + NaNO₂(s) → HNO₂(l) + NaCl(s) 2NaCl(s) + H₂O(l) → 2HCl(g) + Na₂O(s) ∆H° = -507.1 kJ/mol NO(g) + NO₂(g) + Na₂O(s) → 2NaNO₂(s) ∆H° = -427.0 kJ/mol NO(g) + NO₂(g) → N₂O(g) + O₂(g) ∆H° = -43.01 kJ/mol 2HNO₂(l) → N₂O(g) + O₂(g) + H₂O(l) ∆H° = +34.02 kJ/mol the enthalpy of reaction for the given reaction is -39.1 kJ/mol.
We can use the given reactions and their enthalpies to find the enthalpy of reaction for the given equation.
The given equation is:
HCl(g) + NaNO₂(s) → HNO₂(l) + NaCl(s)
We can break down this reaction into several steps using the given reactions as follows:
Na₂O(s) + 2HCl(g) → 2NaCl(s) + H₂O(l) (reverse of the given reaction)
∆H° = +507.1 kJ/mol (reverse the sign of given reaction)
2NaCl(s) + H₂O(l) → Na₂O(s) + 2HCl(g)
∆H° = -507.1 kJ/mol (given reaction)
NO(g) + NO₂(g) + Na₂O(s) → 2NaNO₂(s)
∆H° = -427.0 kJ/mol (given reaction)
2HNO₂(l) → N₂O(g) + O₂(g) + H₂O(l)
∆H° = +34.02 kJ/mol (given reaction)
NO(g) + NO₂(g) → N₂O(g) + O₂(g)
∆H° = -43.01 kJ/mol (given reaction)
Now, we can add these equations and their enthalpies to get the overall enthalpy of the given reaction:
HCl(g) + NaNO₂(s) → HNO₂(l) + NaCl(s)
H₂O(l) + 2NaCl(s) → Na₂O(s) + 2HCl(g) ∆H° = -507.1 kJ/mol
Na₂O(s) + NO(g) + NO₂(g) → 2NaNO₂(s) ∆H° = -427.0 kJ/mol
2HNO₂(l) → N₂O(g) + O₂(g) + H₂O(l) ∆H° = +34.02 kJ/mol
NO(g) + NO₂(g) → N₂O(g) + O₂(g) ∆H° = -43.01 kJ/mol
HCl(g) + NaNO₂(s) → HNO₂(l) + NaCl(s) ∆H° = -39.1 kJ/mol
Learn more about enthalpy here:
https://brainly.com/question/13996238
#SPJ1
HELPPPPP (100 POINTS)
Assume that the water stream is replaced by a stream of CCl4. Predict what would happen in each case.
a. charged acetate strip:
b. charged vinyl strip:
c. Explain your predictions.
Answers
Answer:c
Explanation:c
If 335 g of water at 65.5 °C loses 9750 J of heat,
what is the final temperature of the water? Liquid
water has a specific heat of 4.18 J/(g*°C).
Answers
Answer:
We can use the formula for heat lost by a substance to calculate the final temperature:
Q = m * c * ΔT
where Q is the heat lost, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
In this case, we know the values of Q, m, and c, and we need to find ΔT. Rearranging the formula, we have:
ΔT = Q / (m * c)
Substituting the given values, we get:
ΔT = 9750 J / (335 g * 4.18 J/(g*°C)) ≈ 6.9 °C
Therefore, the final temperature of the water is:
65.5 °C - 6.9 °C ≈ 58.6 °C
So the final temperature of the water is approximately 58.6 °C.
The final temperature of the water is approximately 58.5°C.
To find the final temperature of the water, we first need to understand that the heat lost by the water is calculated using the formula q = mcΔT, where 'q' is the Heat Transfer, 'm' is the mass of the water, 'c' is the specific heat of the water, and 'ΔT' is the change in temperature.
First, rearrange the formula to find ΔT = q/(mc).
Then, insert the given values (q = -9750 J, m = 335 g, c = 4.18 J/g°C).
The negative sign denotes heat loss.
You will find ΔT is approximately -7°C.
This is the amount the temperature decreases.
Subtract ΔT from the initial temperature of the water (65.5°C - 7°C), to get the final temperature of approximately 58.5°C.
Learn more about Heat Transfer here:
https://brainly.com/question/34419089
#SPJ2
More than two thirds of elements are classified as
Answers
Answer:
Metals
Explanation:
Two Thirds means 75 %.
Metals account for about more than two - thirds of all elements.
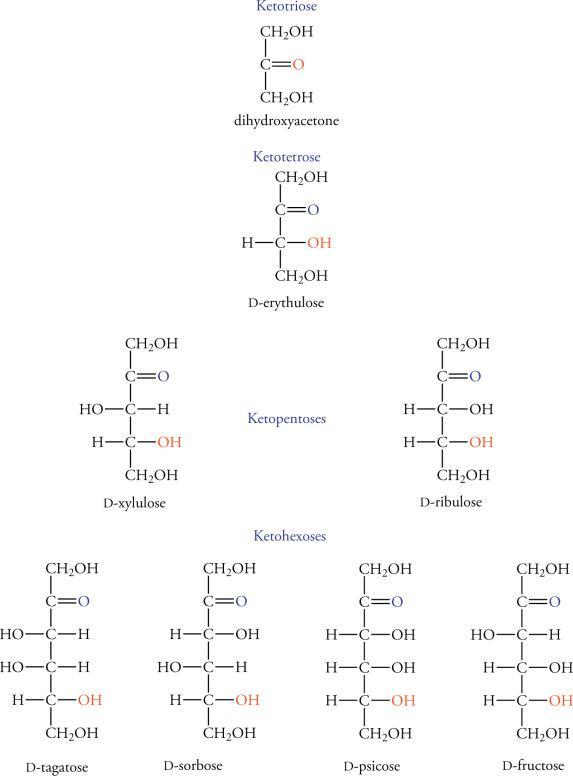
1. List the ketose carbon(s) that is(are) reduced with NaBH4.
Answers
Answer:
ehh im nit sure but here!
Explanation:
Sputnik is the Russian name for __________.
Answers
A meteor falls toward Earth's surface. Given that the acceleration due to
gravity is 9.8 m/s2, what is the meteor's potential energy if it has a mass of 50
kg at an altitude of 300 m²
A. 147,000 u
B. 16 J
C. 22,050,000 J
D. 1,531 J
Answers
Answer:
E = 147000 J
Explanation:
Given that,
The mass of meteor, m = 50 kg
The altitude of the meteor, h = 300 m
We need to find the potential energy of the meteor. The formula for the potential energy is given by :
\(E=mgh\)
Put all the values,
\(E=50\times 9.8\times 300\\\\E=147000\ J\)
So, the required potential energy is equal to 147000 J.
the ATOMIC WEIGHT OF ALUMINUM (AL) is 26.98. It has 14 neutrons. Aluminum has ?
a. an atomic mass of 13
b. an atomic number 13
c. 26 electrons
d. 26 protons
I believe b. an atomic number 13. is the answer.
Answers
The atomic weight of Aluminum (AL) is 26.98. It has 14 neutrons. Aluminum has an atomic number 13. Therefore, option B is correct.
What is atomic number?The atomic number of an element represents the number of protons in the nucleus of its atoms. Since the atomic number of an element is unique and determines its position in the periodic table, aluminum has an atomic number of 13.
The number of electrons in a neutral atom is equal to the number of protons, which in this case is 13. Therefore, aluminum has 13 electrons.
The atomic weight of aluminum (Al) is 26.98, which represents the weighted average mass of all the naturally occurring isotopes of aluminum, taking into account their relative abundances. The number of neutrons in an atom of aluminum is given as 14.
Thus, the correct option is B.
To learn more about the atomic number, follow the link:
https://brainly.com/question/16858932
#SPJ3
If a less concentrated initial solution of sodium bicarbonate was used in beaker C, would that solution require more or less bicarbonate to neutralize the acid? Why?
Answers
If a less concentrated initial solution of sodium bicarbonate was used in beaker C, it would require more bicarbonate to neutralize the acid.
What is concentrated?Concentrated means something that has been increased in strength or power by reducing its volume. It can refer to a solution that has a higher concentration of solutes than the original solution, a sound that is louder or stronger, or a force that is more powerful or intense. Concentrated can also refer to a person’s focus or attention on one particular thing, when their thoughts and energy are directed to a single point.
This is because the concentration of sodium bicarbonate determines how much of the acid can be neutralized by the solution. If the initial solution is less concentrated, then it will take more of the bicarbonate to neutralize the same amount of acid.
To learn more about concentrated
https://brainly.com/question/30558048
#SPJ1
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL.
Answers
Answer:
Molality = 8.57 m
Explanation:
Given data:
Molarity of solution = 5.73 M
density = 0.9327 g/mL
Molality of solution = ?
Solution:
Molality = moles of solute / kg of solvent.
Kg of solvent:
Mass of 1 L solution = density× volume
Mass of 1 L solution = 0.9327 g/mL × 1000 mL
Mass of 1 L solution = 932.7 g
Mass of solute:
Mass of 1 L = number of moles × molar mass
Mass = 5.73 mol × 46.068 g/mol
Mass = 263.97 g
Mass of solvent:
Mass of solvent = mass of solution - mass of solute
Mass of solvent = 932.7 g - 263.97 g
Mass of solvent = 668.73 g
In Kg = 668.73 /1000 = 0.6687 Kg
Molality:
Molality = number of moles of solute / mass of solvent in Kg
Molality = 5.73 mol / 0.6687 Kg
Molality = 8.57 m
Considering the definition of molality , you obtain that the molality of a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL is 8.57 \(\frac{moles}{kg}\).
Molality is the ratio of the number of moles of any dissolved solute to kilograms of solvent.
The Molality of a solution is determined by the expression:
\(Molality=\frac{number of moles of solute}{kilogramof solvent}\)
You have a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL.
Molarity is the number of moles of solute that are dissolved in a certain volume.
In this case, taking into account that the volume considered is 1 L, the number of moles of solute is 5.73 moles.
On the other side, density is the ratio of the weight (mass) of a substance to the volume it occupies. So, being 1 mL= 0.001 L, 0.9327 g/mL means that you have 0.9327 grams per 1 mL or 932.7 g per 1 L.
So, being the mass of solution calculated as number of moles multiplied by the molar mass, and being the mass of the solution 932.7 grams in 1 L, the mass of water is:
Mass of solvent = mass of solution - mass of solute
Mass of solvent = mass of solution - number of moles× molar mass
Mass of solvent = 932.7 g - 5.73 mol× 46.068 g/mol
Mass of solvent = 932.7 g - 263.97 g
Mass of solvent = 668.73 g= 0.66873 kg
Then, the molality can be calculated as:
\(Molality=\frac{5.73 moles}{0.66873 kg}\)
Solving:
molality= 8.57 \(\frac{moles}{kg}\)
Finally, the molality of a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL is 8.57 \(\frac{moles}{kg}\).
Learn more about:
density: brainly.com/question/952755?referrer=searchResults brainly.com/question/1462554?referrer=searchResults molalitybrainly.com/question/20366625?referrer=searchResults brainly.com/question/4580605?referrer=searchResults molarity with this example: brainly.com/question/15406534?referrer=searchResultsPLSS HELPP ME i dont knowww

Answers
Answer:
non polar. polar ionic substance
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
the iupac name of the compound

Answers
Answer:
3-Pentyn-1-ol
Explanation:
tripple bond is at 3 postion from alochol
carbon are 5 atoms so pent
yn becauese its alkyne
What is the reason for the trend in Electron Affinity? Select all that apply.
A. number of protons in the nucleus
B. number of energy levels around the nucleus C. number of neutrons in the nucleus
D. larger atomic mass number
E. number of valence electrons.
Answers
Answer:
i think it a and d
Explanation: