Answers
Explanation:
A displacement reaction is the one wherein the atom or a set of atoms is displaced by another atom in a molecule. For instance, when iron is added to a copper sulphate solution, it displaces the copper metal. A + B-C → A-C + B.
Related Questions
Question Completion Status:
QUESTION 1
The smallest difference beteen two objects that can be faithfully detected by a measuring device is:
O calibration
quantification
O resolution
O accuracy
O standarization
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Answers
The smallest difference between two objects that can be faithfully detected by a measuring device would be resolution.
Other options are incorrect because:
Calibration refers to the manipulations made to measuring instruments in order to increase their accuracies.Quantification refers to the act of valuing/quantifying something by attaching a figure to it.Accuracy refers to the closeness of measurements to their true values.Standardization refers to the manipulation of instruments to make them conform to acceptable standards.Resolution, on the other hand, refers to the smallest measurable distance between two points or objects. Hence, the correct option is resolution.
More on resolution can be found here: https://brainly.com/question/13115101
A mass of 1.71 g pure barium hydroxide is transferred quantitatively to a 250 cm3
volumetric flask and made up to the mark with distilled water. Using a pipette, 25.0 cm3
of the barium hydroxide solution are placed in a conical flask and a few drops of methyl
orange indicator are added. Hydrochloric acid is added slowly from a burette until the
endpoint is reached. The titre value is 12.6 cm3
What will the colour change of the indicator at the endpoint be?
Answers
The methyl orange indicator will change from red to yellow at the titration's endpoint.
How can the color of the indicator's change at the terminus be determined?An acid-base indicator called methyl orange changes color between the pH ranges of 3.1 and 4.4. In acidic and basic solutions it is red and yellow, respectively
In this instance, an acid, hydrochloric acid, is being used to titrate the barium hydroxide solution. The pH of the solution will fall as we add the acid since it will neutralize the base. The hue of the methyl orange indicator will vary when the pH ranges from 3.1 to 4.4
All of the barium hydroxide will have interacted with the hydrochloric acid by the time the titration is complete leaving a neutral solution. When the methyl orange indicator becomes yellow the solution's pH is in the basic range.
Therefore, the methyl orange indicator will change from red to yellow at the titration's endpoint.
Learn more about acid-base indicator here: brainly.com/question/1918667
#SPJ1
Problem PageQuestion Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O. Suppose 3.3 g of hydrochloric acid is mixed with 5.00 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Round your answer to 2 significant digits.
Answers
Answer:
Mass of Nacl = 4.92 gram (Approx)
Explanation:
Given:
Hydrochloric acid = 3.3 g
Sodium hydroxide = 5 g
Computation:
Hcl + NaoH ⇒ Nacl + H₂O
Number of mole of Hcl = 3.3 / 36.46 = 0.0905 moles
Number of mole of NaoH = 5 / 40 = 0.125 moles
We, know that number of moles in Hcl is less then number of mole in NaoH
So,
Number of mole of Hcl = Number of mole of Nacl
So,
Mass of Nacl = Number of mole of Nacl × Molar mass of Nacl
Mass of Nacl = 0.0905 × 54.4
Mass of Nacl = 4.92 gram (Approx)
grams in 3500mL of Hydrogen sulfide
Answers
The molar mass of hydrogen sulfide is 34.1 g/mol.
What is the formula for converting molarity to grams?To begin, rearranging the equation to determine the number of moles in this solution. No. Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.
The molar mass of NaCl is 58.44 g/mol. We may now utilize the rearranged equation. (g) = No. 1 x 58.44 = 58.44 g. Moles (mol) × Molar Mass (g/mol) = 1 x 58.44 = 58.44 g.
1 milliliter = 1 gram of water. The weight of the other components varies. 1 mL of milk equals 1.04 g, 1 mL of flour = 0.53 g, and 1 mL of sugar equals 0.85 g.
The concentration represents the solution’s mass concentration, which is represented in density units (often g/l or g/ml). Molar mass is the mass of one mole of a substance.
To learn more about convert molarity to refer:
https://brainly.com/question/14416031
#SPJ1
Which describes a likely advantage of the roots that gymnosperms have?
-They are shallow, preventing the plants from growing too tall above ground.
-They are shallow, allowing the plants to easily gather water from Earth’s surface.
-They grow deep, preventing the plants from growing too tall above the ground.
-They grow deep, allowing the plants to gather water from far below Earth’s surface.
please answer quickly! im being timed on EDGE
Answers
Answer: They grow deep, allowing plants to gather water from far below Earth's surface- D
Explanation: Sorry I dont have time to explain its D on edgen
To solve this we must be knowing each and every concept related to gymnosperms. Therefore, the roots that gymnosperms grow deep, allowing the plants to gather water from far below Earth’s surface. The correct option is option D.
What is gymnosperms?Unlike angiosperms, and flowering plants, because seeds are closed by fully developed ovaries, or fruits, gymnosperms, or any vascular plant, reproduce by the use of a exposed seed, or ovule.
Many gymnosperms bear their seeds in cones, which are known as "seeds" because they are never visible until they are fully developed. The roots that gymnosperms grow deep, allowing the plants to gather water from far below Earth’s surface.
Therefore, the roots that gymnosperms grow deep, allowing the plants to gather water from far below Earth’s surface. The correct option is option D.
To know more about gymnosperms, here:
https://brainly.com/question/4526473
#SPJ6
What is the limiting reactant and the theoretical yield of copper obtained when 286.2g of copper (I) oxide reacts with 262.2g of copper (I) sulfide. The unbalanced equation in is Cu2O + Cu2S -> Cu + SO2
If the actual yield is 370.8g, what is the percent yield?
How much of the excess reagent remains?
Answers
A. The limiting reactant is copper (I) oxide
B. The theoretical yield of copper is 384 grams
C. The percent yield would be 96.56%
D. The amount of excess reagent that remains would be 103 grams.
Stoichiometric problemsThe balanced equation of the reaction is as follows: \(2Cu_2O + Cu_2S -- > 6Cu + SO_2\)
The mole ratio of the reactants is 2:1.
Mole of 286.2 g of copper (I) oxide = 286.2/143 = 2 mol
Mole of 262.2 g of copper (I) sulfide = 262.2/159 = 1.65 mol
Going by the mole ratio, copper (I) oxide is limiting. The actual amount of copper (I) sulfide required would be:
1/2 x 1 = 1 mol
Thus, excess copper (I) sulfide = 1.65 - 1 = 0.65 mol
0.65 mol = 0.65 x 159 = 103 grams
To get the theoretical yield of copper: mole ratio of copper (I) oxide and copper produced = 1:3
2 mole of copper (I) oxide = 6 moles of copper
6 mol copper = 6 x 64 = 384 grams
If the actual yield is 370.8 grams:
Percent yield = 370.8/384 x 100
= 96.56%
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
What does wave frequency measure?
A. the distance between two corresponding points on ALTERNATING waves
B. the number of waves that pass through a fixed point in a certain amount of time
C. the distance between two corresponding points on ADJACENT waves
D. the height of the wave in relation to the center line
Answers
The frequency is used to measure the number of waves that pass through a fixed point in a certain amount of time. That is option B.
What is frequency of a wave?A wave is defined as a disturbance in a medium that travel from a point to another in an organised fashion.
There are different properties of a wave that include the following:
amplitude, wavelength, frequency, and speedThe frequency of a wave is defined as the property of a wave that shows the the number of crests (high points) of waves that pass the fixed point in a given number of time.
Therefore, frequency of a wave measures the number of waves that pass through a fixed point in a certain amount of time.
Learn more about frequency here:
https://brainly.com/question/27151918
#SPJ1
Wave frequency measures the waves that pass through a fixed point in a certain amount of time (option B)
What is wave frequency?Frequency of a wave is defined as the number of complete oscillations, vibrations or waves made in 1 second. This is written formula:
Frequency (f) = Number of oscillation (n) / time (s)
f = n / t
The SI unit of wave frequency is Hertz.
Wave frequency is related to speed of wave and wavelength according to the following formula:
Velocity (v) = wavelength (λ) × frequency (f)
v = λf
Wave frequency is also related to the period of a wave according to the following formula:
Frequency (f) = 1 / Period (T)
f = 1 / T
With the above information about wave frequency, we can conclude that the correct answer to the question is option B
Learn more about frequency:
https://brainly.com/question/18125929
#SPJ1
4. In which pair of substances does the first underlined atom have a lower oxidation number than the second? A. NH3OH+ NH4– B. H2O H2O2 C. SO3 SO42– D. HCHO C
Answers
Answer:
Option B is correct.
Only this option has the first underlined element with a lower oxidation number than the second amongst the options.
Explanation:
Complete Question
In which pair of substances does the first underlined atom have a lower oxidation number than the second?
A. NH₃OH⁺, NH₄⁻ (N is underlined)
B. H₂O, H₂O₂ (O is underlined)
C. SO₃, SO₄²⁻ (S is underlined)
D. HCHO, C (C is underlined)
Solution
In determination of the oxidation number of an atom in a compound, we first name the unknown oxidation number x.
Then, the total oxidation number of the atoms in the compound is equal to the charge of on the compound (or radical).
So, elements in their neutral state have no charge and no oxidation number.
A. NH₃OH⁺, NH₄⁻ (N is underlined)
N in NH₃OH⁺
Oxidation number of N = x
Oxidation number of H = +1
Oxidation number of O = -2
x + (3×+1) + (-2) + (+1) = +1
x - 3 - 2 + 1 = 1
x = +5
N in NH₄⁻
Oxidation number of N = x
Oxidation number of H = +1
x + (4×1) = -1
x + 4 = -1
x = -1 - 4 = -5
First underlined element has a greater oxidation number than the second. So, this doesn't qualify.
B. H₂O, H₂O₂ (O is underlined)
O in H₂O
Oxidation number of H = +1
Oxidation number of O = x
(2×1) + x = 0
2 + x = 0
x = -2
H₂O₂
Oxidation number of H = +1
Oxidation number of O = x
(2×1) + (2×x) = 0
2 + 2x = 0
2x = -2
x = (-2/2) = -1.
First underlined element has a lower oxidation number than the second. So, this qualifies.
C. SO₃, SO₄²⁻ (S is underlined)
S in SO₃
Oxidation number of S = x
Oxidation number of O = -2
x + (3×-2) = 0
x - 6 = 0
x = +6
SO₄²⁻
Oxidation number of S = x
Oxidation number of O = -2
x + (4×-2) = -2
x - 8 = -2
x = 8 - 2 = +6
First underlined element has the same oxidation number as the second. So, this doesn't qualify.
D. HCHO, C (C is underlined)
C in HCHO
Oxidation number of H = +1
Oxidation number of C = x
Oxidation number of O = -2
+1 + x + 1 - 2 = 0
x = 0
C in C
Oxidation number of C = x
x = 0
First underlined element has the same oxidation number as the second. So, this doesn't qualify.
Hope this Helps!!!
What did Josh do that allowed him to dissolve that much sugar in only 100 g of water?
Josh used sugar cubes instead of granulated sugar.
Josh used a pan with a large surface area.
Josh stirred the solution.
Josh heated the water.
Answers
Answer:
heated the water
Explanation:
What will be the charge of the ion formed from each of these atoms ?
Si14, As33, Mg12, Rb37, F9, Ge32, Sn50
Answers
Answer:
Si14- Si^4+
As33- As^3-
Mg12- Mg^2+
Rb37- Rb^+
F9- F^-
Ge32- Ge^4+
Sn50- Sn^2+, Sn^4+
Explanation:
The elements shown in the answer have their common ions written beside them.
Silicon mostly forms positive ions in oxyacids and complex ions. Arsenic mostly forms its anion. Magnesium forms only the +2cation just as rubidium only forms the +1 cation. The fluoride ion is F^- while tin may for a +2*or +4 cation. Germanium usually forms the +4 cation.
Which portion of a molecule of F2O has partial positive charge?
Question 3 options:
A)
The F atoms
B)
The central O atom
C)
The partial charge on each atom is zero
D)
The partial charge on each atom is negative
Answers
The partial charges on each fluorine atom are negative. Option B) The central O atom is the correct answer. Option B
The partial charges in a molecule are determined by the electronegativity values of the atoms involved. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In the case of \(F_2O\), fluorine (F) is more electronegative than oxygen.
Fluorine is the most electronegative element on the periodic table, meaning it has a high ability to attract electrons. Oxygen is also relatively electronegative but less so than fluorine. When fluorine atoms bond with oxygen, the shared electrons will be pulled more towards the fluorine atoms, creating a polar covalent bond.
In \(F_2O\), each fluorine atom will pull the shared electrons towards itself, resulting in a higher electron density around the fluorine atoms. This creates a region of partial negative charge around the fluorine atoms.
Conversely, the oxygen atom will have a region of lower electron density and, therefore, a partial positive charge. This is because the shared electrons spend more time around the fluorine atoms due to their higher electronegativity.
Option B
For more such question on partial charges visit:
https://brainly.com/question/29974793
#SPJ8
When the light energy from sunlight hits matter, _________.
A. heat energy is formed
B. nothing happens
C. potential energy is formed
Answers
Answer:
When the light energy from sunlight hits matter, heat energy is formed
Answer:
A
Explanation:
when light energy hit matter heat energy formed
Use the name to write the formula for the following ionic compound: scandium (III) hydroxide
Use the name to write the formula for the following ionic compound: titanium (IV) cyanide
Answers
Answer:
.
Explanation:
.
2 NH3 + 3 CuO g 3 Cu + N₂ + 3H₂O
In the above equation how many moles of N₂ can be made when 38 moles of CuO are
consumed?
Answers
The moles of N2 formed when 38 moles of CuO is consumed 12.66 mol.
What is moles?
A mole of a substance can be defined as an amount of substance having 6.022 x 10^23 particles in it. This number is known as Avogadro's number. All the balanced chemical equations are written using number of moles of reactants and products.
The balanced chemical equation of the reaction can be written as,
2 NH3 + 3 CuO ------> 3 Cu + N2 + 3 H2O
Here, 2 moles of ammonia is reacting with 3 moles of CuO to give 3 moles of Cu, 1 mole of N2 and 3 moles of water. So when 38 moles of CuO is consumed,
Moles of N2 = 38 x 1 / 3 = 12.66 mol.
Therefore, 12.66 mole of N2 is formed when 38 moles of CuO is consumed in the reaction.
To learn more about moles click on the given link https://brainly.com/question/14357742
#SPJ1
Kevin travels from the United States to Australia for the winter, but when he gets there it feels more like summer. Which statement explains this?
A) The Northern Hemisphere is experiencing an equinox.
B) The Southern Hemisphere is experiencing an equinox.
C) The Southern Hemisphere is tilted toward the sun, so it is summer in Australia.
D) The Southern Hemisphere is tilted away from the sun, so it is summer in Australia.
Answers
Answer:
The Northern Hemisphere is tilted away from the sun.
Explanation:
Kevin travels from the United States to Australia for the winter, but when he gets there it feels more like summer. Which statement explains this? The Northern Hemisphere is tilted away from the sun. When it is winter, the United States receives the least direct solar energy, as compared with the rest of the year.
Hello! Allow me to help you :)
The answer is B) The Southern Hemisphere is experiencing an equinox.
What is an Equinox?
The two days of the year on which neither hemisphere is tilted toward or away from the sun.
This means what?
This means that if he goes to the Northern Hemisphere it will be warm. And if he goes to the Southern, he will be warm too.
~*Answer*~
The Southern Hemisphere is experiencing an equinox.
Reasoning:
Kevin travels from the United States to Australia for the winter, but when he gets there it feels more like summer. Which statement explains this? The Northern Hemisphere is experiencing an equinox. The Southern Hemisphere is experiencing an equinox. The Southern Hemisphere is tilted toward the sun, so it is summer in Australia.
Therefore, your answer is B) The Southern Hemisphere is experiencing an equinox.
If this helped then..
Give it a thanksGive it a good rating :)And maybe brainliest!
This has been..
~NatLikesAnime~
#LearningWithBrainly
Which could be the missing reason in Step 3?
alternate interior angles are congruent
alternate exterior angles are congruent
vertical angles are congruent
corresponding angles are congruent
Answers
Answer:
a) alternate interior angles are congruent
Explanation:
on edgen
Although Na and p are present in the same period yet their oxides are different in nature
Answers
Although sodium and phosphorus are both found in the same period of the periodic table, the nature of their oxides is different. Although P2O5 is acidic, Na2O is basic.
What type of oxides are there in different groups and eras?Metal oxides are basic, whereas non-metal oxides are acidic. The metallic character of the elements lessens as you move from left to right over time, while the non-metallic character grows.
Which oxide naturally has a higher acidity?Since the oxides in an element's higher oxidation state are more acidic than those in its lower oxidation state, dinitrogen pentoxide is the most acidic oxide compared to nitrogen dioxide and nitrogen tetraoxide.
To know more about acidic visit:-
https://brainly.com/question/14072179
#SPJ1
1. Calculate the number of moles of oxygen atoms present in 1.50 mol of barium sulfate.
Answers
Answer:
\(n_O=6.00molO\)
Explanation:
Hello.
In this case, since the molecular formula of barium sulfate is:
\(BaSO_4\)
Which has four oxygen atoms, we can also say that one mole of barium sulfate has four moles of oxygen; in such a way, the moles of oxygen atoms in 1.50 moles of barium sulfate are:
\(n_O=1.50molBaSO_4*\frac{4molO}{1molBaSO_4} \\\\n_O=6.00molO\)
Best regards.
Qué uso le darías en la vida diaria ayuda
Answers
Answer:
ako po kailangan ko po nga ayuda
Explanation:
thanks for the points ❤️
The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed? Report your answer to 1 decimal place.
Answers
Answer:
12.5g
Explanation:
Half life = 2.4 Minutes.
The half life of a compound is the time it takes to decay to half of it's original concentration or mass.
Time lapsed= 7.2 minutes. This is equivalent to 3 half lives ( 3 * 2.4)
Initial mass = 100g
First half life;
100g --> 50g
Second half life;
50g --> 25g
Third half life;
25g --> 12.5g
The amount of Zn-71 that remains after 7.2 mins has elapsed is 12.5 g
We'll begin by calculating the number of half-lives that has elapsed. This can be obtained as follow:
Half-life (t½) = 2.4 mins
Time (t) = 7.2 mins
Number of half-lives (n) =?\(n = \frac{t}{t_{1/2}} \\\\n = \frac{7.2}{2.4} \\\\\)
n = 3Thus, 3 half-lives has elapsed.
Finally, we shall the amount remaining. This can be obtained as follow:Original amount (N₀) = 100 g
Number of half-lives (n) = 3
Amount remaining (N) =?\(N = \frac{N_{0}}{2^{n}} \\\\N = \frac{100}{2^{3 }}\\\\N = \frac{100}{8}\\\\\)
N = 12.5 gThus, the amount of Zn-71 that remains after 7.2 mins is 12.5 g
Learn more: https://brainly.com/question/9561011
(b) Calculate the pH of the following buffer solution.
A solution of 10.0g each of formic acid (CH COOH (Mwt 46g/mol) and Potassium formate (CKHCOO
(Mwt 84g/mol) dissolved in 1.0 litre of water.
(formic acid ka = 1.8 x 104)
Answers
The pH of the buffer solution can be determined using Henderson equation. The pH is obtained as 4.51.
What is buffer solution?A buffer solution is a solution with constant pH and its maintains the pH of a solution upto a desired point. The pH of a buffer solution is determined using Henderson equation as written below:
pH = Pka + ln [A-]/[HA]
Where, [A-] be the concentration of the salt and [HA ] be that of the acid.
Given that 10 g of acid and its salt are dissolved in 1 L solution. Thus,
no.of moles of acid = 10 g / 46 = 0.217 in L means 0.217 M
concentration of salt = 10 g/ 84 g in one L = 0.119 M.
ka = 1.8 × 10⁴
pka = log Ka = 4.25
pH = 4.25 + ln (0.119 / 0.217)
= 4.51
Therefore, the pH of the solution is 4.51.
To find more on buffer solution, refer here:
https://brainly.com/question/24262133
#SPJ1
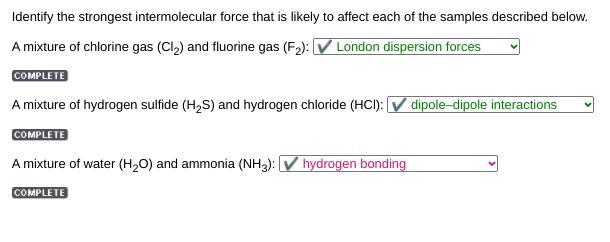
Identify the strongest intermolecular force that is likely to affect each of the samples described below.
A mixture of chlorine gas (Cl) and fluorine gas (F): V London dispersion forces
COMPLETE
Tweaks
Menu
A mixture of hydrogen sulfide (H2S) and hydrogen chloride (HCI): V dipole-dipole interactions
Search
Selection
COMPLETE
Guess
this
hydrogen bonding
A mixture of water (H2O) and ammonia (NH3):
Answers
Answer:
A mixture of chlorine gas (Cl2) and fluorine gas (F2):
✔ London dispersion forces
Explanation:
4Na + O2 2Na2O
How many moles of Na2O will be produced from 4.35 x 10^24 atoms of Na?
with explanation
Answers
N
a
+
O
2
→
2
N
a
2
O
.
By the stoichiometry of this reaction if 5 mol natrium react, then 2.5 mol
N
a
2
O
should result.
Explanation:
The molecular mass of natrium oxide is
61.98
g
⋅
m
o
l
−
1
. If
5
m
o
l
natrium react, then
5
2
m
o
l
×
61.98
g
⋅
m
o
l
−
1
=
154.95
g
natrium oxide should result.
So what have I done here? First, I had a balanced chemical equation (this is the important step; is it balanced?). Then I used the stoichiometry to get the molar quantity of product, and converted this molar quantity to mass. If this is not clear, I am willing to have another go
C D
Which level of organization is pictured?
O cell
O tissue
O organ
O organ system
Answers
What mass of MnO2 is produced when 445 grams of H2O are reacted?
H20 + 2MnO4+ Br- + BrO3 + 2MnO2 + 20H-
Answers
Answer:
when 445 grams of H2O are reacted, 4,300 grams (or 4.3 kilograms) of MnO2 are produced.
Explanation:
The balanced chemical equation for the reaction is:
16H+ (aq) + 2MnO4- (aq) + 10Br- (aq) → 2MnO2 (s) + 5Br2 (aq) + 8H2O (l)
According to the equation, 2 moles of MnO4- react to produce 2 moles of MnO2. Therefore, we need to find out the number of moles of MnO4- that react with the given amount of H2O and then use stoichiometry to calculate the number of moles of MnO2 produced.
First, let's calculate the number of moles of H2O:
mass of H2O = 445 g
molar mass of H2O = 18.015 g/mol
number of moles of H2O = mass/molar mass = 445 g/18.015 g/mol = 24.7 mol
From the balanced equation, we can see that 1 mole of H2O reacts with 2 moles of MnO4-. Therefore, the number of moles of MnO4- required to react with 24.7 mol of H2O is:
number of moles of MnO4- = 2 × number of moles of H2O = 49.4 mol
Since 2 moles of MnO4- produce 2 moles of MnO2, we can say that 1 mole of MnO4- produces 1 mole of MnO2. Therefore, the number of moles of MnO2 produced is also 49.4 mol.
Finally, we can calculate the mass of MnO2 produced:
mass of MnO2 = number of moles of MnO2 × molar mass of MnO2
mass of MnO2 = 49.4 mol × 86.94 g/mol = 4,300 g
Therefore, when 445 grams of H2O are reacted, 4,300 grams (or 4.3 kilograms) of MnO2 are produced.
Name the functional group in the
following molecule:
CH3CH2OCH2CH3
A ester
B. acid anhydride
C ketone
D. ether
Answers
Answer:
d
Explanation:
i just did it
The functional group in the following molecule CH₃CH₂OCH₂CH₃ is ether.
What is functional group?Functional group is defined as a substituent or group of atoms or an atom which causes chemical reactions.Each functional group will react similarly regardless to the parent carbon chain to which it is attached.This helps in prediction of chemical reactions.
The reactivity of functional group can be enhanced by making modifications in the functional group .Atoms present in functional groups are linked to each other by means of covalent bonds.They are named along with organic compounds according to IUPAC nomenclature.
Functional group inter conversion is also possible by retro -synthesis.In some cases , functional groups can be charged molecules.
Learn more about functional group,here:
https://brainly.com/question/14618322
#SPJ7
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Match the terms with their definitions
Frequency, Amplitude, Crest, Wavelength, Medium, Trough
Matter distributed by energy
Highest point of transverse wave
Lowest point of transverse wave
Distance between midpoint of wave and crest
Distance traveled in one wave cycle
Number of waves that pass per second
(from brainpop waves.) PLease HeLP (Worth 25 POINTS)
Answers
Answer:
amplitude= distance between midpoint of a wave and crest
crest= highest point of transverse wave
wavelength= distance traveled in one wave cycle
medium= matter distributed by energy
trough=lowest point of transverse wave
frequency=number of waves that pass per second
Explanation:
A wave is a dynamic perturbation of one or even more quantities that propagates in a specific medium.
What is wave?A wave is a dynamic perturbation of one or even more quantities that propagates in physics, mathematics, or related sciences. When waves oscillate frequently around an equilibrium position at a certain frequency, they are said to be periodic. A traveling wave is one in which the whole waveform moves inside one direction; in contrast, a standing wave is one in which two periodic waves are overlaid and move in the opposing directions.
In a wave form, there are some points in which the wave amplitude seems reduced or even zero, and these positions have null vibration amplitudes. A wave equation or perhaps a one-way wave equation describing single wave propagation inside a specific direction is frequently used to describe waves.
amplitude= distance between midpoint of a wave and crest
crest= highest point of transverse wave
wavelength= distance traveled in one wave cycle
medium= matter distributed by energy
trough=lowest point of transverse wave
frequency=number of waves that pass per second
Therefore, a wave is a dynamic perturbation of one or even more quantities that propagates
To know more about wave, here:
https://brainly.com/question/13779567
#SPJ3
A fish company delivers 15 kg of salmon 4.7 kg Of crab and 1.76 kg of oysters to your seafood restaurant what is the total mass in kilograms of the seafood
Answers
Answer:
21.46 kg.
Explanation:
Hello!
In this case, when a variety of measurements are said to contribute to a total, we understand we need to add them all up in order to get that total; in such a way, since the given seafood is composed by 15 kg of salmon, 4.7 kg of crab and 1.76 kg of oysters, the total mass turns out:
\(m_T=15kg+4.7kg+1.76kg\\\\m_T=21.46kg\)
Best regards!
O2 is which of the following?
Both compound and molecule
One atom
compound
Molecule
Answers
The chemical formula O₂ which is oxygen is a molecule as it is made up of 2 oxygen atoms.
What is chemical formula?
Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ1