Answers
Explanation:
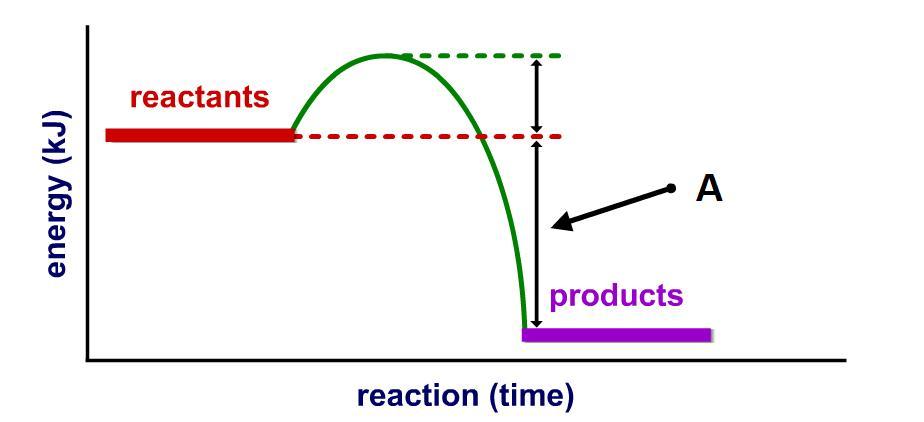
A is the difference of energy of products and reactants. This difference is called enthalpy.
So we can see that the value of products is lower than the value of the energy of the reactants, which means that the result will be negative.
Answer: C. -VE
Related Questions
When the equation below is balanced, what is the coefficient for ammonia gas?
_NH3(g) + _O2(g) → _N2(g) + _H2O(g)
Answers
Answer:
4NH3(g) + 3O2(g) → 2N2(g) + 6H2O(g)
Explanation:
the half-life of uranium-232 is 69 years. If the nuclear byproduct contains uranium-232, it is not considered safe for human contact until 99.2% of it has been decayed. How long will this take
Answers
After 1 year, then,
x = 0.5/69 = 0.00724
0.724% of uranium-239 has been decayed.
To find how long it will take for 99.2% of the element to decay, we can use a similar method:
x years/0.992 = 69 years/0.5
x = 136.896 years
Therefore, it will take 136.896 years for 99.2% of the nuclear byproduct to have decayed.
Let me know if you have any questions
It will take approximately 480 years for 99.2% of uranium-232 to decay.
The formula of radioactive half-life = \(\frac{N}{N₀} = (\frac{1}{2})^{n}\)
Where N = amount of isotope remaining
N₀ = original amount of isotope
n = number of half-lives
From the given values:
N = 100 5 - 99.2%
N = 0.8 % = 0.008
N₀ = 100% = 1
Substituting to find n
0.008/1 = (1/2)ⁿ
㏒₁₎₂0.008 = n
n = ㏒0.008/㏒(1/2)
n = 6.96
Therefore, number of half-lives n = 6.96
One half-life = 69 years
6.96 half-lives = 6.96 * 69 = 480.24 years
Therefore, it will take approximately 480 years for 99.2% of uranium-232 to decay.
learn more at: https://brainly.com/question/17070257
a fisherman in a boat is drinking a of hot coffee. the large lake below his boat is full of cold water. which statement is an accurate comparison of the lake water and the coffee?
Answers
Answer:
see below
Explanation:
The lake will have more total thermal energy but the particles in the coffee will be moving faster.
A comparison of hot coffee and cold lake is, the heat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Conservation of energy
The principle of conservation of energy states that the total energy of an isolated system is always conserved.
Heat lost by the hot coffee = Heat absorbed by the cold lake
Heat transfer processHeat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Thus, a comparison of hot coffee and cold lake is, the heat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Learn more about heat transfer here: https://brainly.com/question/12072129
Which of these is an example of a physical property?
A. Chlorine oxidizes bacterial cells.
B. Chlorine gas is yellow-green in color.
C. Chlorofluorocarbons (CFC's) react with ozone (O₂).
D. Potassium ignites when placed in water.
Answers
Answer:
B. Chlorine gas is yellow-green in color.
How many molecules are in a 0.00583 mole sample of H₂O?
Answers
Answer:
3.51 x 10²¹ molecules H₂O
Explanation:
To find the amount of molecules in the sample, you need to multiply the amount of moles by Avogadro's Number. Avogadro's Number is a ratio comparing the amount of molecules per every 1 mole. It is important to arrange the ratio in a way that allows for the cancellation of units (moles should be in the denominator). The final answer should have 3 sig figs like the given value (0.00583 = 3 sig figs).
Avogadro's Number:
6.022 x 10²³ molecules = 1 mole
0.00583 moles H₂O 6.022 x 10²³ molecules
-------------------------------- x -------------------------------------- = 3.51 x 10²¹ molecules
1 mole
Are neutrons inside the nucleus ?
Answers
Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom's mass. ... The neutron was discovered in 1932 by the English physicist James Chadwick.
Hope this helps : )
Hope this helps!
Also please can I have the brainiest?!
DIFFERENCE BETWEEN LOWEST AND HIGHEST MELTING POINT OOF CARBON FIBRE
Answers
Answer:
The carbon fiber itself will withstand incredibly high temps. The weak point will be the epoxies or the resins.Ambient-cured epoxies will typically begin breaking down around 100 °C. The heat-cured epoxies that will go up to around 300 °C. As you get into the high temp ratings, the epoxies get trickier to handle, install, cure.Since the compositions of most irons while casting are around the eutectic point (lowest liquid point) of the iron–carbon system, the melting temperatures usually range from 1,150 to 1,200 °C.As is evident, at such high temperatures, the epoxies of Carbon Fibers will melt. So, they are not yet used for iron casting.
Rank the following gases from by density at 1.00 atm and 298 K. Rank from most dense to least dense. To rank items as equivalent, overlap them. Reset Help More dense Less dense N20 02 HF CH4
Answers
Ranking the given gases at 1.00 atm and 298k, from most dense to least dense;
\(N_{2}O > O_{2} > HF > CH_{4}\)
Density:
We use the word "density" to indicate how much space (or "volume") an object or substance occupies in relation to the amount of matter contained therein (it's mass). Density can also be defined as the quantity of mass per unit of volume. A dense object is one that is both hefty and small.
Mathematically,
D = M/V
Where,
D is density,
M is mass and
V is volume.
Given,
Pressure = 1 atm
Temperature = 298 K
We know that from the Universal gas equation,
PV = nRT
V = nRT/P
V = constant
∴ The density of the gases
D ∝ M
We know that,
\(M_{N_{2}O }= 44.013 g/mol\)
\(M_{O_{2} }=32g/mol\)
\(M_{HF}=20.01g/mol\) and
\(M_{CH_{4} }= 16.04g/mol\)
Hence,
Ranking the given gases at 1.00 atm and 298k, from most dense to least dense;
\(N_{2}O > O_{2} > HF > CH_{4}\)
Learn more about Density here
https://brainly.com/question/1354972
#SPJ4
MATCH THE NAMES OF THE MICROSCOPE PARTS WITH THEIR DECRIPTIONS
Answers
The Microscope part and their right descriptions are as follows
Iris Diaphragm: A. Increases or decreases the light intensity
Objective Lens System: B. After light passes through the specimen, it next enters this lens system
Stage: C. Platform that supports a microscope slide
Adjustment Knob: D. Causes stage (or objective lens) to move upward or downward
Condenser: E. Concentrates light onto the specimen
what other parts of microscope parts and their description should you know?Other parts of a microscope and their description that you should know about includes;
Eyepiece - The lens that you look through to see the image of the specimen.
Body tube - The tube that connects the eyepiece to the objective lenses.
Arm - The part of the microscope that supports the body tube and connects it to the base.
Base - The part of the microscope that supports the arm and provides stability.
Illuminator - The light source that provides light for the microscope.
Stage clips - The clips that hold the microscope slide in place on the stage.
Revolving nosepiece - The part of the microscope that holds the objective lenses and allows them to be rotated into place.
The above answer is in response to the full question below;
Match the names of the microscope parts in column A with the descriptions in column B. Place the letter of your choice in the space provided.
1. Iris diaphram
2. Objective lens system
3. Stage
4. Adjustment knob
5. Condenser
Increases or decreases the light intensity
2. After light passes through the specimen, it next enters this lens system
3. Platform that supports a microscope slide
4. Causes stage (or objective lens) to move upward or downward
5. Concentrates light onto the specimen
Find more exercises on Microscope;
https://brainly.com/question/1869322
#SPJ1
hi how do i do d (ii)? thanks!

Answers
A solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
We must first convert the amount of nitrate(V) ions in drinking water to moles per litre since the threshold concentration over which "Blue-Baby" Syndrome might manifest is 0.05 mg/cm3.
It is necessary to know the molar mass of nitrate(V) ions in order to convert from mg/cm3 to moles per litre (NO3-).
The formula below can be used to determine NO3-'s molar mass:
N: 14.01 g/mol for the atomic mass
O: 16.00 g/mol is the atomic mass (3 oxygen atoms in NO3-)
NO3-'s total molar mass is equal to 14.01 + 16.00 x 3 = 62.01 g/mol.
Let's now translate the specified concentration from mg/cm3 to moles/liter (M).
As 1 g = 1000 mg, 1 mg/cm3 is 0.001 g/cm3.
1 cm3 = 0.001 g/mL multiplied by 0.001 g/cm3
1 L/1000 mL x 0.001 g/mL = 0.001 g/L
We must change the grams into moles using the molar mass in order to get the molarity:
1.61 × 10-5 mol/L = 0.001 g/L / 62.01 g/mol
As a result, a solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
To know more about Blue-Baby" Syndrome:
https://brainly.com/question/31992990
#SPJ1
2. How many moles of HCI are needed to react with 0.47 g of NaHC03 ?
Answers
HCl reacts with NaHCO₃ this way:
\(HCl_{}+NaHCO_3\rightarrow NaCl+H_2O+CO_2\)The equation is already balanced, it means that 1 mole of HCl reacts with 1 mole of NaHCO₃.
Convert the mass of NaHCO₃ to moles using the molecular mass of NaHCO₃ which is 84g/mol.
\(0.47g\cdot\frac{1mol}{84g}=0.0056mol\)Now, use the ration stated before to find how many moles of HCl react with this amount of NaHCO₃:
\(0.0056mol\cdot\frac{1mol}{1mol}=0.0056mol\)It means that 0.0056 moles of HCl are needed to react with 0.47g of NaHCO₃.
The fossilized remains of a plant were found at a construction site. The fossilized remains contain 1/32 the amount of carbon-14 that is present in a living plant.
Determine the approximate age of these fossilized remains.
Answers
Answer:
28645 years
Explanation:
Given the formula;
0.693/t1/2 = 2.303/t log (No/N)
Given that N = 1/32 No
Note;
t1/2 = half life of Carbon-14
t = time required for N amount of carbon -14 to remain= 5,730 years
No= amount of carbon 14 initially present
N = amount of carbon-14 after time t
Substituting values;
0.693/5,730 = 2.303/t log (No/1/32No)
0.693/5,730 = 2.303/t log 32
1.21 * 10^-4 = 3.466/t
t = 3.466/1.21 * 10^-4
t = 28645 years
Any preserved imprints, remains and traces of once a living organism from history are called fossils. By utilizing the radioactive property of organic compounds one can determine the age of an object.
The approximate age of the fossilizes remain is 28645 years.
This can be estimated as:
The formula used will be:
\(\dfrac{0.693}{\dfrac{t1}{2}}= \dfrac{2.303}{t} log (\dfrac{No}{N})\)
Where,
Half-life of Carbon-14 =\(\dfrac{t1}{2}\) Time required (t) for N amount of carbon -14 to remain= 5,730 yearsAmount of carbon 14 initially present = \(N_{o}\)Amount of carbon-14 after time t = NGiven,
N = \({\dfrac{1}{32} No\)
Replacing values in formula:
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} log \:({{\dfrac{No}{\dfrac{1}{32} No}})\)
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} \;log 32\)
\(1.21 \times 10^{-4} = \dfrac{3.466}{t}\)
\(t = \dfrac{3.466}{1.21} \times 10^{-4}\)
\(t = 28645 \:\text{years}\)
Therefore, the approximate age of the fossil is 28645 years.
To learn more about fossils and their age follow the link:
https://brainly.com/question/8917701
Compound A decomposed to form compound B and c I. A first order reaction at 250c° the rate constants for the reaction is 0.45,what is the half life of compound A ?
Answers
The half-life of a reaction is the time it takes for the concentration of a reactant to decrease to half its initial value. In a first-order reaction, the rate of the reaction is directly proportional to the concentration of the reactant. This means that the rate of the reaction depends on the concentration of compound A.
To calculate the half-life of compound A, you can use the equation:
half-life = (ln(2)) / k
where k is the rate constant for the reaction.
Plugging in the values given in the question, we get:
half-life = (ln(2)) / 0.45
This simplifies to:
half-life = 1.44 / 0.45
Which gives us a final result of:
half-life = 3.2 hours
So the half-life of compound A at 250°C is approximately 3.2 hours.
Learn more about Half-life reactions here: https://brainly.com/question/14228544
What is the molarity of a 3.00 L solution that contains 0.400 mol FeCl3?
1.20 M
0.266 M
7.50 M
0.133 M
Answers
Answer: 0.133 mol/l
Explanation: molality = concentration = 0.400 mol/3.0 l
Phosphorus-32 (32P) is an isotope that is commonly used for medical and biological research. Phosphorus-32 has a half-life of 14.28 days. A researcher orders a sample of 32P that has an activity of 5.0 mCi when it arrives. If delivery of the sample took 48 hours, what was the activity of the sample when it was shipped from the manufacturer
Answers
The activity of the sample when it was shipped from the manufacturer is 4.54 mCi
How to determine the number of half-lives that has elapsedFrom the question given above, the following data were obtained:
Time (t) = 48 hoursHalf-life (t½) = 14.28 days = 14.28 × 24 = 342.72 hours Number of half-lives (n) =?n = t / t½
n = 48 / 342.72
n = 0.14
How to determine the activity of the sample during shipping Number of half-lives (n) = 0.14Original activity (N₀) = 5.0 mCiActivity remaining (N) =?N = N₀ / 2ⁿ
N = 5 / 2^0.14
N = 4.54 mCi
Thus, the activity of the sample during shipping is 4.54 mCi
Learn more about half life:
https://brainly.com/question/2674699
What is the molarity of a 450 mL solution containing 3.5 moles of potassium nitrate?
How many moles of sodium nitrate are in 0.33 L of a 0.33 M solution?
What volume of 2.6 M potassium chlorate (PClO3 molar mass= 123 g/mol) contains 48 g of solute?
What mass of ammonia (NH3) is contained in 1500 mL of 0.75 M solution?
A 2.0 M HCl has a volume of 800 mL. What is the mole value?
What is the mass percent of magnesium acetate solution made with 34 g of solute and 150 g of water?
A 325 g sample of a sodium fluoride solution contains 15 g of solute. Determine the mass percent of the solute.
What is the total mass of a solution when the mass of the solute is 17g and the mass percent of the solute is 42%?
Answers
1. The molarity of the solution is 7.78 M. 2. There are 0.109 moles of sodium nitrate in 0.33 L of a 0.33 M solution.
2. We are given 3.5 moles of potassium nitrate in 450 mL of solution. To find molarity, we need to convert mL to L:
450 mL = 0.45 L
Then we can use formula:
Molarity = moles of solute/liters of solution
Molarity = 3.5 moles / 0.45 L= 7.78 M
2. We are given volume of 0.33 L and a molarity of 0.33 M for sodium nitrate. To find the number of moles of sodium nitrate, using:
moles of solute = molarity x liters of solution
moles of solute = 0.33 M x 0.33 L = 0.109 moles
To know more about sodium nitrate, here
brainly.com/question/13073227
#SPJ1
--The complete Question is 1. What is the molarity of a 450 mL solution containing 3.5 moles of potassium nitrate?
2. How many moles of sodium nitrate are in 0.33 L of a 0.33 M solution? --
What is the name of the ion with 4 neutrons and 4 electrons with a 1- charge?
A: Hydrogen
B: Berrylium
C: Lithium
D: Helium
Answers
Answer:
C.
Explanation:
I search it on Gøogle
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
2) What is the pH when the concentration of [H*] is 4.22 x 108
3) What is the pH when the pOH is 7
4) What is the concentration of [OH¹¹] when the pH is 4.3
5) What does it mean to be diprotic?
6) What does amphoteric mean?
7) WHat can you use to measure pH?
8) WHat does a buffer solution do?
9) What does titration do?
10) What is the difference between a strong acid and a weak acid?
Answers
2. The pH is 4.22 × 10⁸. 3. pH is 7, 4. pOH is 9.7, 5. diprotic is explained below, 6. Amphoteric is explained below, 7. pH meter, 8. A buffer solution is explained below, 9. Titration is explained below, 10. Difference between strong and weak acids is explained below.
2. pH = -log[H⁺]
pH = -log(4.22 x 10⁻⁸)
pH ≈ 7.375
3. pH + pOH = 14
pH + 7 = 14
pH = 14 - 7
pH = 7
4. pH + pOH = 14
pOH = 14 - pH
pOH = 14 - 4.3
pOH ≈ 9.7
Now,
[H⁺] × [OH⁻] = 1.0 x 10⁻¹⁴
[OH⁻] = 1.0 x 10⁻¹⁴ / [H⁺]
[OH⁻] = 1.0 x 10¹⁴ / 10^(-pOH)
[OH⁻] = 1.0 x 10⁻¹⁴ / 10^(-9.7)
[OH⁻] ≈ 1.99 x 10⁻⁶ M
5. Being diprotic means that a molecule or ion can donate or release two protons (H⁺ ions) in an acid-base reaction.
6. Amphoteric refers to a substance that can act as both an acid and a base.
7. The pH can be measured using a pH meter or a pH indicator.
8. A buffer solution is a solution that can resist changes in pH when small amounts of acid or base are added to it.
9. Titration is a laboratory technique used to determine the concentration of a solution by reacting it with a solution of known concentration (titrant) of another substance.
10. A strong acid is an acid that completely ionizes in water, releasing all of its hydrogen ions. A weak acid is an acid that does not completely dissociate into ions when dissolved in water.
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ1
Use the periodic table to select the element from the drop-down menu that has the correct relative electronegativity.
yo its been 3 minutes where my answer at
Mg>
P>
C >
Br>
Answers
The correct answer based on relative electronegativity would be:
Br > P > C > Mg
What is Electronegativity?
Electronegativity is a measure of an element's tendency to attract a bonding pair of electrons towards itself when it forms a chemical bond with another element. It is a property of elements that reflects their ability to attract and hold onto electrons in a chemical bond.
Electronegativity is a measure of an element's tendency to attract a bonding pair of electrons. In general, electronegativity increases from left to right across a period in the periodic table and decreases from top to bottom within a group. Therefore, Bromine (Br) would have the highest electronegativity among the given options, followed by Phosphorus (P), Carbon (C), and Magnesium (Mg) with the lowest electronegativity.
Learn more about Electronegativity from the given link
https://brainly.com/question/24977425
#SPJ1
What are some convincing reasons for researcher's to choose your biome ( P.S. this is for a project) pls reply as soon as you can
Answers
Choosing a biome for a research project can provide a wealth of opportunities to better understand the natural world and contribute to important conservation and management efforts.
Why is biome important in research?There are several convincing reasons for researchers to choose a particular biome for their project, some of which include:
Biodiversity: Biomes are characterized by their unique plant and animal species, which can provide a diverse range of research opportunities, including studying their adaptations, behaviors, and interactions.
Climate Change: Biomes are also influenced by climate change, making them an important area of research to better understand how these ecosystems are being affected by changes in temperature and precipitation patterns.
Human Impacts: Human activities, such as deforestation, agriculture, and urbanization, can have a significant impact on biomes. Studying these impacts can help researchers better understand how to mitigate and manage these effects.
Biogeochemical Cycles: Biomes are also important for studying biogeochemical cycles, such as the carbon and nitrogen cycles, which are essential for sustaining life on Earth.
Conservation: Finally, studying biomes can also contribute to conservation efforts, helping to preserve these unique ecosystems and the species that inhabit them.
Find out more on biome here: https://brainly.com/question/2096327
#SPJ1
If the length of a wave is doubled, what will happen to the value of the frequency? (c = λv) A. it will increase B. it will decrease C. it will stay the same
Answers
Answer:
B
Explanation:
If the frequency is doubled, the wavelength is only half as long.
If the wavelength Doubled, the frequency is reduced.
calculate the molar internal energy of carbon dioxide at 298.15k , taking it's translational and rotational degrees of freedom into consideration
Answers
Answer:
Explanation:
To calculate the molar internal energy of a gas at a given temperature, you need to know the molar specific heat capacities at constant volume and constant pressure for the gas. These values are typically provided in tables of thermodynamic data, which can be found in various sources such as textbooks or online. Since you mentioned that you want to take the translational and rotational degrees of freedom into consideration, you will need to use the molar specific heat capacity at constant volume, which accounts for these degrees of freedom.
Once you have the molar specific heat capacity at constant volume for the gas, you can use the equation U = Cv * T, where U is the molar internal energy, Cv is the molar specific heat capacity at constant volume, and T is the temperature in kelvins. In your case, the temperature is 298.15 K, so plugging in the appropriate values and solving for U will give you the molar internal energy of carbon dioxide at that temperature.
It's important to note that the molar specific heat capacity at constant volume is typically a function of temperature, so you will need to use the appropriate value for the temperature you are interested in. Additionally, different sources may provide slightly different values for the molar specific heat capacity, so it's always a good idea to consult multiple sources to get a sense of the range of possible values.
An electron has a
charge.
Answers
An electron has a negative charge.
The charge of an electron is a fundamental property of the particle, and it is denoted by the symbol "e." The magnitude of the charge of an electron is approximately 1.602 × 10^-19 coulombs (C). This value is considered the elementary charge and is used as a reference for other charges. The charge of an electron plays a significant role in determining the behavior and interactions of atoms and molecules. It is opposite in sign to the charge of a proton, which is positive. The electron's charge allows it to interact with other charged particles, such as protons and ions, through electrostatic forces. Electrons are subatomic particles that orbit the nucleus of an atom in specific energy levels or orbitals. They contribute to the overall stability and chemical properties of atoms and participate in chemical bonding and reactions. The movement of electrons between atoms is what enables the formation of chemical bonds and the sharing or transfer of electrons to create ions. In summary, the charge of an electron is negative, and it plays a fundamental role in the structure and behavior of atoms and molecules.
for more questions on electron
https://brainly.com/question/26084288
#SPJ8
shown below is the reaction of an alkene with an electrophile. reaction for the mechanism step below, draw curved arrows to show electron reorganization. use the arrow tool to specify the origin and the destination of the reorganizing electrons. consult the arrow-pushing i
Answers
The mechanism of the reaction of an alkene with an electrophile attached below
There's 2 steps in reaction between alkene and electrophile
an electrophilic attackThe K in the KI electrophile is attacked by the two pi electrons from the double bond during the first step of the process, which is denoted by a curved arrow. The hydrogen from HBr and a carbon from the double bond combine to produce a C-H sigma bond thanks to the two pi electrons. In order to create a halide anion, the electrons from the H-X bond simultaneously transfer to the halogen. One of the carbons becomes an intermediate carbocation with an electron deficit when pi electrons from the double bond are removed. The positive charge is housed in an unhybridized p orbital on this sp2 hybridized carbon atom.
Nucleophilic attack by halide anion.In order to receive an electron pair from the nucleophilic halide anion, the generated carbocation can now function as an electrophile. The neutral alkyl halide is the end result of electrophilic addition, and the electron pair transforms into an X-C sigma bond.
The HBr, HCl, HI, and HF halides can all take part in this reaction and add on in the same way. Although various halides do react at varying rates, this is because the H-X bond weakens with increasing X due to inadequate orbital overlap.
Your question is incomplete but most probably your full question attached below
Learn more about electrophile attack at https://brainly.com/question/14704243
#SPJ4


i need the answers to this chemistry quiz

Answers
9. The number of mole of the argon contained in the tank is 2.39 moles
10. The volume (in liters) of H₂O produced from the reaction is 73.5 liters
9. How do i determine the number of mole?The number of mole of the argon contained in the tank can be obtained as follow:
Volume of balloon (V) = 6.25 LPressure (P) = 9.4 atmTemperature (T) = 26 °C = 26 + 273 = 299 KGas constant (R) = 0.0821 atm.L/mol KNumber of mole (n) =?PV = nRT
Inputting the given parameters, we have:
9.4 × 6.25 = n × 0.0821 × 299
Divide both sides by (0.0821 × 299)
n = (9.4 × 6.25) / (0.0821 × 299)
n = 2.39 moles
Thus, the number of mole of the argon gas in the tank is 2.39 moles
10. How do i determine the volume of H₂O produced?The volume of H₂O produced can be obtained as shown below:
4NH₃(g) + 5O₂(g) -> 6H₂O(g) + 4NO(g)
From the balanced equation above,
4 liters of NH₃ reacted to produced 6 liters of H₂O
Therefore,
49 liters of NH₃ will react to produce = (49 × 6) / 4 = 73.5 liters of H₂O
Thus, the volume of H₂O produced is 73.5 liters
Learn more about number of mole:
https://brainly.com/question/29927685
#SPJ1
Freezing point depression is a colligative property.The freezing point of pure water is 0.0°C. How many grams of ethylene glycol (C2H6O2) must be mixed in 100.0 g of water to lower the freezing point of the solution to -4.3°C?_______ g
Answers
Explanation:
The freezing point depression is a colligative property. We have to find the mass of ethylene glycol that we have to add to 100.0 g of water to change its freezing point from 0.0 °C to -4.3 °C.
The freezing point depression for a solution can be calculated using the following equation:
ΔTf = kf * molality * i
Where ΔTf is the freezing point depression, kf is the freezing point depression constant (it depends on the solvent and for water is 1.86 °C/m), molality is the molality of the solution and i is the Van't Hoff factor.
The Van't Hoff factor represents the number of particles formed when that compound dissolves. In our case the solute is ethylene glycol, a covalent compound, so it won't form ions when dissolved in the water. Then i is equal to 1.
The temperature must change from 0.0 °C to -4.3 °C, then the freezing point depression is 4.3 °C. So we know that:
i = 1 kf = 1.86 °C/m ΔTf = 4.3 °C
We can replace those values in the formula and find the molality of the solution.
ΔTf = kf * molality * i
4.3 °C = 1.86 °C/m * molality * 1
molality = 4.3 °C/(1.86 °C/m)
molality = 2.31 m
Now we can get the moles of ethylene glycol from the definition of the molal concentration. Molality are the moles of solute per kg of solvent. The mass of water is 100 g.
mass of solvent = 100.0 g * 1 kg/(1000 g)
mass of solvent = 0.100 kg
molality = moles of solute/(mass of solvent in kg)
moles of solute = molality * mass of solvent in kg
moles of solute = 2.31 m * 0.100 kg
moles of solute = 0.231 moles
And finally we can convert the moles of ethylene glycol to grams using its molar mass.
molar mass of C₂H₆O₂ = 62.07 g/mol
mass of C₂H₆O₂ = 0.231 moles * 62.07 g/mol
mass of C₂H₆O₂ = 14.4 g
Answer: 14.4 g of ethylene glycol must be mixed.
Which of the following elements is most reactive?
A.LI
B.Ne
C.K
D.Au
E.Cs
Answers
Answer:
E
Explanation:
Anything going down Column 1 is going to be more reactive than anything else.
So the only contenders that are in column 1 are Li and Cs. The most reactive ones are the ones going down.
Cs is at the bottom of column 1. The answer is E
find the sum of 15, 9, 3, ....... 45
Answers
Answer:
hope this helps
Explanation:
72
the rate of decomposition of acetaldehyde, ch3cho(g) , into ch4(g) and co(g) in the presence of i2(g) at 800 k follows the rate law rate of reaction
Answers
The catalyst for the reaction is I2 and the step which is slower is second step
The rate law expression shows that the rate of the reaction is directly proportional to the concentration of I2. This indicates that I2 is involved in the rate-determining step of the reaction, which is likely the slowest step in the overall reaction.
From the mechanism given, we can see that the first step involves the reaction between CH3CHO and I2 to form CH3I, HI, and CO. The second step involves the reaction between CH3I and HI to form CH4 and I2. The rate of the overall reaction will be limited by the slower of these two steps.
To determine the slower step, we need to look at the reaction intermediates in each step. In the first step, the intermediate HI is a radical, which is highly reactive and likely to react quickly with other species present in the reaction mixture. In contrast, in the second step, the intermediate CH3I is relatively stable and less likely to react quickly with other species.
Therefore, the slower step is likely the second step, which involves the reaction between CH3I and HI to form CH4 and I2. This step is likely rate-determining and determines the overall rate of the reaction.
Learn more about catalyst here:
https://brainly.com/question/1565029
#SPJ4
the complete question is :
At 800 K, the rate of decomposition of aldehyde (CH3CHO) into CCH and CO follows the rate law: r=K[CCH3CHO][I2]. The decomposition is thought to follow a two-step mechanism:
CH3CHO+I2→CH3I+HI+CHOCH3I+HI→CH4+I2
What is the reaction's catalyst? Which of the two steps is the more difficult?