What is reduced and what is oxidized in the reaction between a monosaccharide and a ferric ion?
Answers
In the reaction between a monosaccharide and a ferric ion, The carbonyl carbon is oxidized to a carboxyl group, the cupric ion is reduced.
Chemical reactions often involve color changes, temperature changes, gas evolution, or precipitate formation. Simple examples of everyday reactions are digestion, combustion, and cooking. The definition of reaction is a reaction. An example of a reaction is someone stopping their car at a stop sign. noun. response to stimuli.
The five basic types of chemical reactions are combination, decomposition, single exchange, double exchange, and combustion. By analyzing the reactants and products of a particular reaction, we can classify them into one of these categories. Some responses fit into multiple categories. It is modeled after the old Italian reaction, the French response, derived from the medieval Latin response (nominative to react). It is a noun of action formed in Late Latin from the past participle stem of the Latin reader.
Learn more about the reaction here
https://brainly.com/question/11231920
#SPJ4
Related Questions
What speed will you have to go from Chicago to Hackensack in 260 hours
Answers
Speed you will have to go from Chicago to Hackensack in 260 hours is 4.9Km/hr.
The pace at which an object's position changes in any direction is referred to as its speed. The distance travelled in relation to the time it took to travel that distance is how speed is defined. Since speed simply has a direction and no magnitude, it is a scalar quantity.Speed = distance / timeUnit of speed is Km/hr or m/sec. Speed is calculated by dividing the distance travelled by the amount of time it took to get there. Divide the distance by the speed to find the passing time. Multiply the speed by the time to find the distance.Given,
Time = 260 hours
distance between Chicago to Hackensack is 1277 km
So speed = 1277/ 260 = 4.9 Km/ hr
Therefore, speed required to go from Chicago to Hackensack in 260 hours is 4.9 Km/ hr.
Learn more about speed here:
https://brainly.com/question/13262646
#SPJ9
How many moles of aluminum ions are present in .40 mole of al2so43
Answers
0.44
⋅
m
o
l
with respect to aluminum sulfate….i.e. a mass of
150.5
⋅
g
...and in this quantity there are TWO EQUIV aluminum….i.e. a mass of
2
×
0.44
⋅
m
o
l
×
27.0
⋅
g
=
23.8
⋅
g
...
Answer:
0.88 ⋅mol of aluminum metal in this molar quantity of salt....
Explanation:
What type of bone is found mostly along the shaft of long bones? a. Marrow b. Compact c. Spongy d. Endosteum
Answers
someone help please i cant figure this out
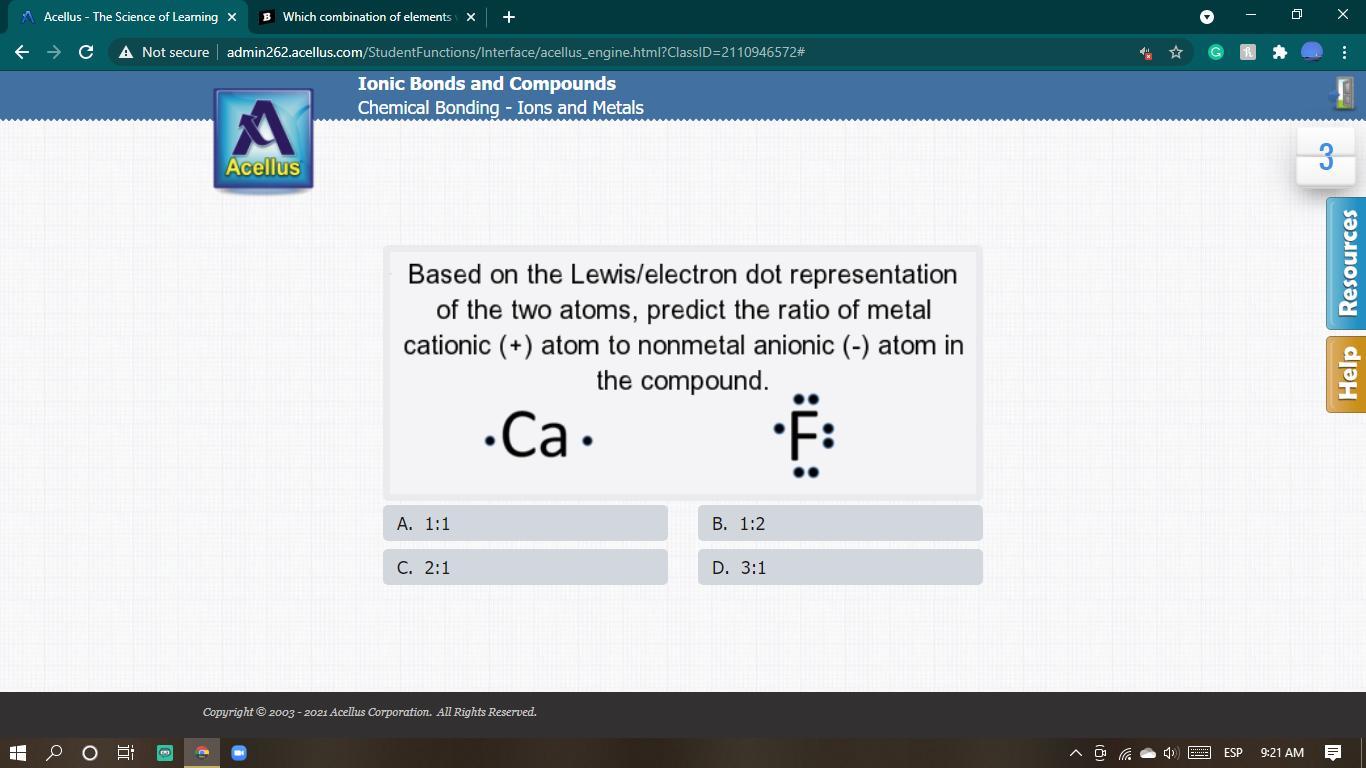
Answers
Answer: 2 : 1
Explanation:
Cation :
Ca - calcium = atomic number = 20
Electron dot configuration : 2, 8, 8, 2
Ca losses 2 electrons in its outermost shell and thus has a charge of 2+ in other to attain a stable octet state.
Anion:
F - has 7 valence electrons and thus needs 1 electron to achieve a stable octet state, hence it accepts one electron has has a charge of (-1)
Therefore,
Ratio of cation (+) to negative (-) = 2 :1
For a process at constant volume, which statement(s) is/are true?
a. q = 0 and ΔE = w
b. w = 0 and ΔE = q
c. ΔE = ΔH
d. q = ΔH
e. w is not equal to 0
Answers
In a constant volume process, no work is done (w = 0) because the volume remains constant. Therefore, option (a) a. q = 0 and ΔE = w, the change in internal energy (ΔE) is equal to the heat transfer (q), resulting in q = 0 is the answer.
b. This statement is not true because it suggests that both work (w) and heat transfer (q) are zero, which is not necessarily the case for a constant volume process.
c. This statement is not necessarily true. ΔE represents the change in internal energy, while ΔH represents the change in enthalpy. Enthalpy is defined as the sum of the internal energy of a system and the product of pressure and volume. In a constant volume process, there is no change in volume, so the change in enthalpy (ΔH) may not necessarily be equal to the change in internal energy (ΔE).
d. This statement is not necessarily true for the same reasons as statement c.
e. This statement is not necessarily true. Work (w) can be zero in a constant volume process if there is no change in pressure, but it can also be non-zero if there are other forms of work involved, such as electrical work or shaft work.
To know more about the first law of thermodynamics refer here :
https://brainly.com/question/3808473#
#SPJ11
how could two objects change in order for there to be a stronger electric force between them?
Answers
Answer:
Increasing the separation distance between objects decreases the force of attraction or repulsion between the objects.
A saturated solution of salt X contains 0. 28g of the salt, in 100cm^3 of solution at 25°C. What is the solubility of the salt X at this temperature(R. M. M of X=56)
Answers
The solubility of salt X at 25°C is 0.05 mol/L.
To calculate the solubility of salt X at 25°C, we need to divide the mass of the salt dissolved in the solution by the volume of the solution.
Given:
Mass of salt X = 0.28 g
Volume of solution = 100 cm^3 = 100 mL
Solubility = Mass of solute / Volume of solution
Since the molar mass (R.M.M) of X is given as 56 g/mol, we can convert the mass of salt X to moles.
Number of moles of salt X = Mass / R.M.M
= 0.28 g / 56 g/mol
= 0.005 mol
Now, we can calculate the solubility:
Solubility = Number of moles / Volume of solution
= 0.005 mol / 100 mL
= 0.05 mol/L
Therefore, the solubility of salt X at 25°C is 0.05 mol/L.
learn more about solubility here
https://brainly.com/question/31493083
#SPJ11
basic forces.
Gravity is one of
Answers
Answer:
Fundamental force, also called fundamental interaction, in physics, any of the four basic forces—gravitational, electromagnetic, strong, and weak—that govern how objects or particles interact and how certain particles decay.
When alkaline hydrolysis was first invented what jobs were people hiring to do?
Answers
When alkaline hydrolysis was first invented, people were hired for various roles related to the process and implementation of this technology. Some of the jobs that emerged include Chemical engineers, Technicians and operators, Waste management specialists, Scientists and researchers.
Chemical engineers: These professionals played a crucial role in developing and optimizing the alkaline hydrolysis process. They were responsible for designing the equipment, developing the necessary chemical reactions, and ensuring the efficient operation of the system.
Technicians and operators: Skilled technicians and operators were hired to operate and maintain the alkaline hydrolysis equipment. They were trained to monitor the process parameters, handle the chemicals involved, and ensure the proper functioning of the system.
Waste management specialists: With the introduction of alkaline hydrolysis as a method for disposal of organic waste, specialized professionals in waste management were employed to oversee the proper handling and treatment of the waste materials. They were responsible for implementing safety protocols, managing waste streams, and complying with environmental regulations.
Scientists and researchers: Alkaline hydrolysis required scientific expertise for continuous improvement and innovation. Scientists and researchers were hired to study the process, analyze the results, and explore potential applications in various fields such as biofuel production and chemical synthesis.
Overall, the introduction of alkaline hydrolysis created employment opportunities for professionals in engineering, chemistry, waste management, and research, among others, as this technology gained recognition and adoption.
To know more about hydrolysis , click here, https://brainly.com/question/31132313
#SPJ11
31.1 grams of O2 and 84.3 grams of F2 are placed in a container with a volume of94.9 L. Find the total pressure if the gasses are at a temperature of 55.77 ° c
Answers
In this question, we have:
31.1 grams of O2
84.3 grams of F2
94.9 L of total volume
55.77°C of temperature which is equal to 328.92 K
Now, to find the pressure of this container, we can find the number of moles of each gas, and add both values together making it one value of moles and then we will use the Ideal gas law to find the pressure, so let's start with O2
The molar mass of O2 is 32g/mol and we have 31.1 grams
32g = 1 mol
31.1g = x moles
x = 0.972 moles of O2
Now for F2, the molar mass is 38g/mol, and we have 84.3 grams
38g = 1 mol
84.3g = x moles
x = 2.22 moles of F2
Now we add these values, 0.972 + 2.22 = 3.192 moles
And now we can use the ideal gas law formula:
PV = nRT
Remember that R is the gas constant, 0.082
P * 94.9 L = 3.192 * 0.082 * 328.92
94.9P = 86.1
P = 0.91 atm
which carbohydrate molecule has the lowest glycemic index?
Answers
The glycemic index (GI) is a measure of how quickly carbohydrates are digested and absorbed by the body, causing a rise in blood sugar levels. The lower the GI, the slower the digestion and absorption of carbohydrates, and the less of an impact they have on blood sugar levels.
Among carbohydrate molecules, fructose has the lowest glycemic index. This is because fructose is a simple sugar that is metabolized differently than other carbohydrates. It is absorbed more slowly in the intestine, and its metabolism does not require insulin. This means that fructose has a minimal effect on blood sugar levels, making it a good choice for people with diabetes or those who want to avoid rapid spikes in blood sugar.
However, it is important to note that consuming large amounts of fructose can have negative health effects, such as contributing to insulin resistance, fatty liver disease, and obesity. Therefore, it is important to consume fructose in moderation and as part of a balanced diet.
To learn more about glycemic index refer to:
brainly.com/question/4130872
#SPJ4
what is the difference between high and low pressure?
A. there are the same number of particle collisions happening in high and low pressure
B. in low pressure, there are more particle collisions happening then high pressure
C. in high pressure, there are more particle collisions then low pressure
D. pressure is not caused by the collisions of particles
Answers
A 3000-gram solution contains 1.5 grams of dissolved NaCl salt. What is the concentration of this solution in ppm
Answers
A 3000-gram solution contains 1.5 grams of dissolved NaCl salt. So, the concentration of this solution is 500 ppm.
Concentration is a measurement of the amount of a substance in a defined space. When talking about concentration, it is often measured in parts per million (ppm). The following statement is the answer to the question below.
A 3000-gram solution contains 1.5 grams of dissolved NaCl salt. The concentration of this solution in ppm is 500 ppm.
The formula for ppm is:
PPM = (mass of solute ÷ mass of solution) × 10⁶
To solve for the concentration of the solution in ppm, we need to use the formula above. Since the question provides the mass of the solute and the mass of the solution, we can just plug them into the formula as shown below:
PPM = (mass of solute ÷ mass of solution) × 10⁶
PPM = (1.5 g ÷ 3000 g) × 10⁶
PPM = (0.0005) × 10⁶
PPM = 500 ppm
Therefore, the concentration of this solution in ppm is 500 ppm.
Learn more about parts per million at https://brainly.com/question/30273212
#SPJ11
4.1 kg of a plastic, used to make plastic bottles, has a carbon footprint of 6.0 kg of carbon dioxide.
Calculate the carbon footprint of one plastic bottle of mass 23.5 g
Answers
Answer:
The carbon footprint of one plastic bottle of mass 23.5 g is 34.390 g.
Explanation:
The carbon footprint of one plastic bottle can be estimated by simple rule of three. That is:
\(x = \frac{23.5\,g}{4100\,g}\times 6000\,g\)
\(x = 34.390\,g\)
The carbon footprint of one plastic bottle of mass 23.5 g is 34.390 g.
If you dissolve 20 mL of flavor crystals into 250 mL of water to make lemonade, what volume of lemonade do you expect to have? Why?
Answers
as the crystals de solved they will not changed the volume of the water as now they are the water
Hawaii, which sits over a hot spot located near the center of the Pacific
plate, is which type of volcano?
Answers
An unidentified flying object has crashed in your yard! An alien emerges from the wreckage. The alien tells you that it has traveled here from the planet Geela which is 5.670x10^3 light years away. How many miles away is Geela? 1.06 x 10^-16 light years = 6.21 x 10^-4 miles
Answers
Answer : Geela away is \(33.22\times 10^{15}\text{ miles}\)
Explanation :
As we are given that the alien tells you that it has traveled here from the planet Geela which is 5.670 × 10³ light years away.
And we know that:
\(1.06\times 10^{-16}\text{ light years}=6.21\times 10^{-4}\text{ miles}\)
Now we have to determine the distance away is Geela.
As, \(1.06\times 10^{-16}\text{ light years}=6.21\times 10^{-4}\text{ miles}\)
So, \(5.670\times 10^{3}\text{ light years}=\frac{5.670\times 10^{3}\text{ light years}}{1.06\times 10^{-16}\text{ light years}}\times 6.21\times 10^{-4}\text{ miles}\)
= \(33.22\times 10^{15}\text{ miles}\)
Therefore, the Geela away is \(33.22\times 10^{15}\text{ miles}\)
I need someones help

Answers
Answer:
y or z one of those two i think its z but im so sorry if im wrong if not z then try y also I learned this last year.
a 0.175-l flask contains cl2 at 25 °c and 475 mm hg. what is the pressure of cl2 if the volume is increased to 0.800 l and the temperature increased to 55 °c?
Answers
Considering the Combined Law Equation, the pressure of Cl₂ if the volume is increased to 0.800 l and the temperature increased to 55 °C is 114.367 mmHg.
Boyle's lawBoyle's law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant: if the pressure increases, the volume decreases while if the pressure decreases, the volume increases.
Mathematically, this law is expressed as:
P×V= constant
Gay-Lussac's lawGay-Lussac's law states that the pressure of a gas is directly proportional to its temperature when the volume is constant: increasing temperature increases pressure, while decreasing temperature decreases pressure.
Mathematically, this law is expressed as:
P÷T= constant
Charles's law
Charles's law states that the volume is directly proportional to the temperature of the gas when the pressure is constant: if the temperature increases, the volume of the gas increases while if the temperature of the gas decreases, the volume decreases.
Mathematically, this law is expressed as:
V÷T= constant
Combined law equation
Combined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law:
(P×V)÷T= constant
Considering an initial state 1 and a final state 2:
(P₁×V₁)÷ T₁= (P₂×V₂)÷ T₂
New pressure
In this case, you know:
P₁= 475 mmHgV₁= 0.175 LT₁= 25 °C= 298 K (being 0 °C= 273 K)P₂= ?V₂= 0.800 LT₂= 55 °C= 328 KReplacing in Combined law equation:
(475 mmHg× 0.175 L)÷ 298 K= (P₂× 0.800 L)÷ 328 K
Solving:
[(475 mmHg× 0.175 L)÷ 298 K] × (328 K÷ 0.800 L)= P₂
114.367 mmHg= P₂
Finally, the new pressure will be 114.367 mmHg.
Learn more about Combined Law Equation:
brainly.com/question/4147359
#SPJ1
if an electron is in a stationary state of an atom, is the electron at rest? if not, what does the term mean?
Answers
No, a stationary state is one that has a specific amount of energy, total angular momentum, and parity; in a stable state, the particle is moving while possessing all of these physical characteristics.
Who or what is an electron?A negative charge subatomic particle known as an electron can either be free or attached to an atom (not bound). Each of the three main types of components within an atom is an atom that is bonded to it; the other three are proton and neutrons.
What is the electron example?The electron, the smallest constituent part of an atom, has a negative surface charge. Protons and electrons are present in an atom in a neutral state in an equal number. The hydrogen ion only possesses one atom or one proton. On the other way, the uranium atom possesses 92 protons, which means 92 electrons.
To know more about Electron visit:
https://brainly.com/question/20513633
#SPJ4
How can nuclear energy be used in medicine
Answers
define the word isotope
Answers
Answer:
Isotope, one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties.
Answer:
Isotopes are two or more types of atoms with the same atomic number but differing nucleon numbers due to varying numbers of neutrons in their nuclei.
OAmalOHopeO
Electrons make up most of the atom's mass. True or false ?
Answers
Answer:
No
Explanation:
Electrons have almost no mass so they cant make up the mass of an atom
In which sealed container would the organisms be able to continuously cycle O2 and CO2 gases?
A.
A terrarium with a frog and a pool of water
B.
An aquarium with water plants and a snail
C.
A terrarium with mice and wood shavings
D.
An aquarium with fish and plastic plants
Answers
In an aquarium with water plants and a snail, organism would be able to continuously cycle O\(_2\) and CO\(_2\) gases. Therefore, the correct option is option B.
What is biological life cycle?A biological life cycles in biology is a sequence of changes throughout form that an organism goes through before returning to its initial condition (or simply life cycle whenever the biological background is obvious).
Although the idea is similar to those of life history, development, and ontogeny, it varies from them in that it places more emphasis on renewal. In an aquarium with water plants and a snail, organism would be able to continuously cycle O\(_2\) and CO\(_2\) gases.
Therefore, the correct option is option B.
To know more about biological life cycle, here:
https://brainly.com/question/1884727
#SPJ9
pressure has little effect on the solubility of liquids and solids because they are almost incompressible.truefalse
Answers
The right answer is true. It is accurate to say that because liquids and solids are almost incompressible, pressure has little impact on their solubility.
We are aware that the concept of compressibility refers to how the study object's qualities can alter simply by applying pressure on it. Whether or not a solid, liquid, or gas can have a solubility that is influenced by the substance's pressure depends on the compressibility factor. We must keep in mind that when we discuss pressure in science, our thoughts must immediately turn to the force per unit area of the substance that is being investigated in each specific example. Because the solid and liquid have a fixed volume while the gas does not, the gas will take on the volume of the container it is carried in, proving that the solid and liquid are less compressible than the gas.
Learn more about compressibility here:
brainly.com/question/23026638
#SPJ4
It is accurate to say that because liquids and solids are nearly incompressible, pressure has minimal impact on their solubility.
Pressure has very little or no impact on how easily particles dissolve in liquid. This is due to the fact that solids and liquids are largely unaffected by changes in pressure due to their incompressibility. The solubility, which depends on gas pressure, is a measurement of the concentration of dissolved gas particles in the liquid. A gas's solubility increases with higher pressure while falling with lower pressure because of an increase in collision frequency. · The solubility of liquids and solids is mostly unaffected by external pressure. The concentration of dissolved gas molecules in the solution at equilibrium is higher at higher pressures because the concentration of molecules in the gas phase rises with increasing pressure.
To know more about solubility click here brainly.com/question/28170449
#SPJ4
What is the correct order of the scientists in order of their work related to the structure of an atom from earliest to most recent?
Neils Bohr, Ernest Rutherford, JJ Thomson, John Dalton, Democritus and Erwin Schrodinger
Erwin Schrodinger, JJ Thomson, John Dalton, Neils Bohr, Ernest Rutherford and Democritus
Democritus, John Dalton, JJ Thomson, Ernest Rutherford, Neils Bohr and Erwin Schrodinger
John Dalton, Neils Bohr, JJ Thomson, Democritus, and Ernest Rutherford and Erwin Schrodinger
PLEASE HELP !!!
Answers
Answer:
C. democritusm John dalton, JJ Thomason, Ernest Rutherford, Neils Bohr, and Erwin Schrodinger.
The correct order of the scientists in order of their work related to the
structure of an atom from earliest to most recent include Democritus, John
Dalton, JJ Thomson, Ernest Rutherford, Neils Bohr, and Erwin Schrodinger.
Democritus was regarded as one of the earliest scientist who first proposed
atomic theory around 460 BC which was then modified and used by others
such as John Dalton, JJ Thomson, Ernest Rutherford and Neils Bohr.
The most recent of them all was Erwin Schrodinger which was the wave
equation for electron movements in the 20th century.
Read more on https://brainly.com/question/20339664
What tool do we use to measure volume
Answers
Answer:
Graduated cylinders, beakers, volumetric pipets, burets and volumetric flasks are five kinds of glassware often used to measure out specific volumes. Volumetric pipets, flasks and burets are the most accurate; the glassware makers calibrate these to a high level of accuracy.
Explanation:
hope this helps :)
write two ionic equation to show that aluminium hydroxide is amphoteric
Answers
Answer:
Explanation:
Aluminium hydroxide is amphoteric. 3 HCl + Al(OH)3 → AlCl3 + 3 H2O. In bases, it acts a Lewis acid by taking an electron pair from the hydroxide ions: Al(OH)3 + OH− → Al(OH)
when 0.083 moles of ammonium sulfate ((nh4)2so4) are dissolved in enough water to make 567 milliliters of solution, how many ammonium ions are present?
Answers
There are 0.83 moles of ammonium ions present in 567 mL of solution.
This can be calculated by first converting the given amount of ammonium sulfate into moles (0.083 moles) and then multiplying it by the number of ammonium ions present in each mole of ammonium sulfate (2). The resulting number is 0.83 moles of ammonium ions. This number can then be converted to milliliters using the molarity equation (M = n/V). This equation can be rearranged to solve for n (the number of moles). Plugging in the values from the given problem yields n = 0.83 moles of ammonium ions present in 567 mL of solution.
To know more about ammonium ions refer to the link brainly.com/question/11173657
#SPJ4
Here’s the second part to my science

Answers
Answer:
"powerhouse"- mitochondria
"stores spare parts"- vacuole
"surrounds the nucleus"- nuclear membrane
"controls the cell"- nucleus
"pathways"- endoplasmic reticulum
"inner layer"- cell membrane
"outer plant covering"- cell wall
"fills up the cell"- ctyoplasm
"collects light"- chloroplasts
"packages proteins"- golgi
"holds info"-chromosomes
"makes protein"-ribosomes
please mark me brainliest if you like my answer <3