what is the amount of iron present in 18.5 g of cereal when the recommended serving size is 30 g? the dri of iron is 18 mg/day. select one:A. 11.10 mgB. 18.0 g C. 1.10 gD 18.0 mg
Answers
The amount of iron present in 18.5 g of cereal is 11.10 mg.
To determine the amount of iron present in 18.5 g of cereal, we need to first find the proportion of iron in a 30 g serving size. If the recommended DRI of iron is 18 mg/day, and we assume the 30 g serving provides this amount, then we can calculate the iron content in the 18.5 g portion.
18.5 g cereal / 30 g serving size = 0.6167
Now, multiply this proportion by the DRI of iron (18 mg/day):
0.6167 * 18 mg = 11.10 mg
Thus, the amount of iron present in 18.5 g of cereal is approximately 11.10 mg.
To know something about iron, click below.
https://brainly.com/question/28675219
#SPJ11
Related Questions
Grams in 1.083e+24
Molecules Na BR
Please help explain why
Answers
Answer:
185 gms o NaBr
Explanation:
Na Br mole weight = 22.989 +79.904 = 102.893 gm/mole
1.083 x 10^24 molecules / 6.022 x 10^23 molecules / mole = 1.798 moles
102.893 * 1.798 = 185 gms
I know this is a lot but I'm really struggling and I need help. Please.
1.
Sodium chlorate decomposes into sodium chloride and oxygen gas as seen in the equation below.
2NaClO3 --> 2NaCl +3O2
How many moles of NaClO3 were needed to produce 61 moles of O2? Round your answer to the nearest whole number.
2. 3 Cu + 8HNO3 g 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of NO can be made when 26 moles of HNO3 are consumed?
3. 3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 75 moles of HNO3 are consumed?
4.
Sodium chlorate decomposes into sodium chloride and oxygen gas as seen in the equation below.
2NaClO3 --> 2NaCl +3O2
How many moles of O2 were produced by 9 moles of NaClO3? Round your answer to the nearest whole number.
5. For the reaction C + 2H2 → CH4, how many grams of carbon are required to produce 16.1 moles of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Carbon 12
6. 3 Cu + 8HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation, how many grams of water can be made when 2.1 moles of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
Answers
1. To determine the number of moles of NaClO₃ required to produce 61 moles of O₂, we can use the stoichiometric ratio between NaClO₃ and O₂.
From the balanced chemical equation, we see that 2 moles of NaClO₃ produce 3 moles of O₂. Therefore, we can set up the following proportion:
2 moles NaClO₃ / 3 moles O₂ = x moles NaClO₃ / 61 moles O₂
Solving for x, we get:
x = (2/3) × (61 moles O₂) / (1 mole NaClO₃) ≈ 41 moles NaClO₃
Therefore, approximately 41 moles of NaClO₃ are needed to produce 61 moles of O₂.
2. From the balanced chemical equation, we see that 3 moles of Cu react with 8 moles of HNO₃ to produce 2 moles of NO. Therefore, we can set up the following proportion:
3 moles Cu / 8 moles HNO₃ = 2 moles NO / x moles HNO₃
Solving for x, we get:
x = (8/3) × (2 moles NO) / (1 mole Cu) ≈ 5.3 moles HNO₃
Therefore, 5.3 moles of HNO₃ are needed to produce 2 moles of NO. If 26 moles of HNO₃ are consumed, we can calculate the moles of NO produced using a proportion:
3. 5.3 moles HNO₃ / 2 moles NO = 26 moles HNO₃ / y moles NO
Solving for y, we get:
y = (2/5.3) × (26 moles HNO₃) / (1 mole NO) ≈ 9.8 moles NO
Therefore, approximately 9.8 moles of NO can be produced when 26 moles of HNO₃ are consumed.
4. From the balanced chemical equation, we see that 8 moles of HNO₃ react with 4 moles of H₂O. Therefore, we can set up the following proportion:
8 moles HNO₃ / 4 moles H₂O = 75 moles HNO₃ / x moles H₂O
Solving for x, we get:
x = (4/8) × (75 moles HNO₃) / (1 mole H2O) = 37.5 moles H₂O
Therefore, 37.5 moles of H₂O can be produced when 75 moles of HNO₃ are consumed.
5. From the balanced chemical equation, we see that 2 moles of NaClO₃ produce 3 moles of O₂. Therefore, we can set up the following proportion:
2 moles NaClO₃ / 3 moles O₂ = 9 moles NaClO₃ / x moles O₂
Solving for x, we get:
x = (3/2) × (9 moles NaClO₃) / (1 mole O₂) = 13.5 moles O₂
Therefore, approximately 14 moles of O₂ are produced when 9 moles of NaClO₃ are used.
6. The stoichiometric ratio between C and CH₄ from the balanced chemical equation is 1:1. This means that 1 mole of carbon is required to produce 1 mole of methane.
Therefore, we can use the following proportion:
1 mole C / 1 mole CH₄
To know more about stoichiometric ratios, visit:
https://brainly.com/question/6907332
#SPJ1
(1.7m - 2) + (2m – 4)
Answers
Answer: 3.7 m − 6
Explanation: Simplify the expression.
brainliest or a thank you pls :)) <3
A battery contains two metals that have different tendencies to attract electrons. If one is lithium with an electron affinity of −3.05, and the other is zinc with an electron affinity of −0.76, describe how the electrons will flow. Then, describe how you could make this an even stronger battery.
Answers
This battery could be made stronger when we make lithium the anode and make zinc the cathode.
What is the electron affinity?We know that the term electron affinity has to do with the fact the a specie is able to attract electrons. Hence, the specie that can be able to attract electrons is said to be have a greater electron affinity.
If we look at the order of the reactivity of the metals, we can see that the lithium has more tendency to exist as a positive ion as such the electron affinity of the lithium atom is very negative and it does not attract electrons.
Learn more about electron affinity:https://brainly.com/question/13646318
#SPJ1
What type of Energy is stored in chemical bonds?
Answers
Answer:
potential energy
Explanation:
Energy, potential energy, is stored in the covalent bonds holding atoms together in the form of molecules. This is often called chemical energy.
A tree rooted into a hillide fall during a torm, the tree roll down the hill at a rate of 13m / how many km/hr i it traveling?
Answers
The speed of the fallen tree from the hill in km/hr would be 46.4 km/hr.
simple unit conversion is applied here so, The problem here is about converting the speed of an object from one unit to another. Specifically, we are to convert from m/s to km/hr.
Speed is the function of distance and time. Thus, the distance needs to be converted to km from m and the time to hr from s.
13 m/s is equivalent to 13 m distance and 1 second time.
remember that:
1000 m = 1 km
13 m = 13/1000
= 0.013 km
3600 seconds = 1 hr
1 second = 1/3600
= 0.00028 hour
The speed of the fallen tree in km/hr will be then:
0.013/ 0.00028 = 46.4 km/hr
To know more about speed click here
brainly.com/question/7359669
#SPJ4
Level 1
What is the first step in completing the
scientific method?
A: Experimentation
B: Forming a hypothesis
C: Analyzing data
D: Making observations
Q1
Level 1
Q2
Which of the following is not true about
a hypothesis?
A: It is an explanation for an observation
B: It must restate the question
1. C. It is testable
D. It can be written as an if/then statement,
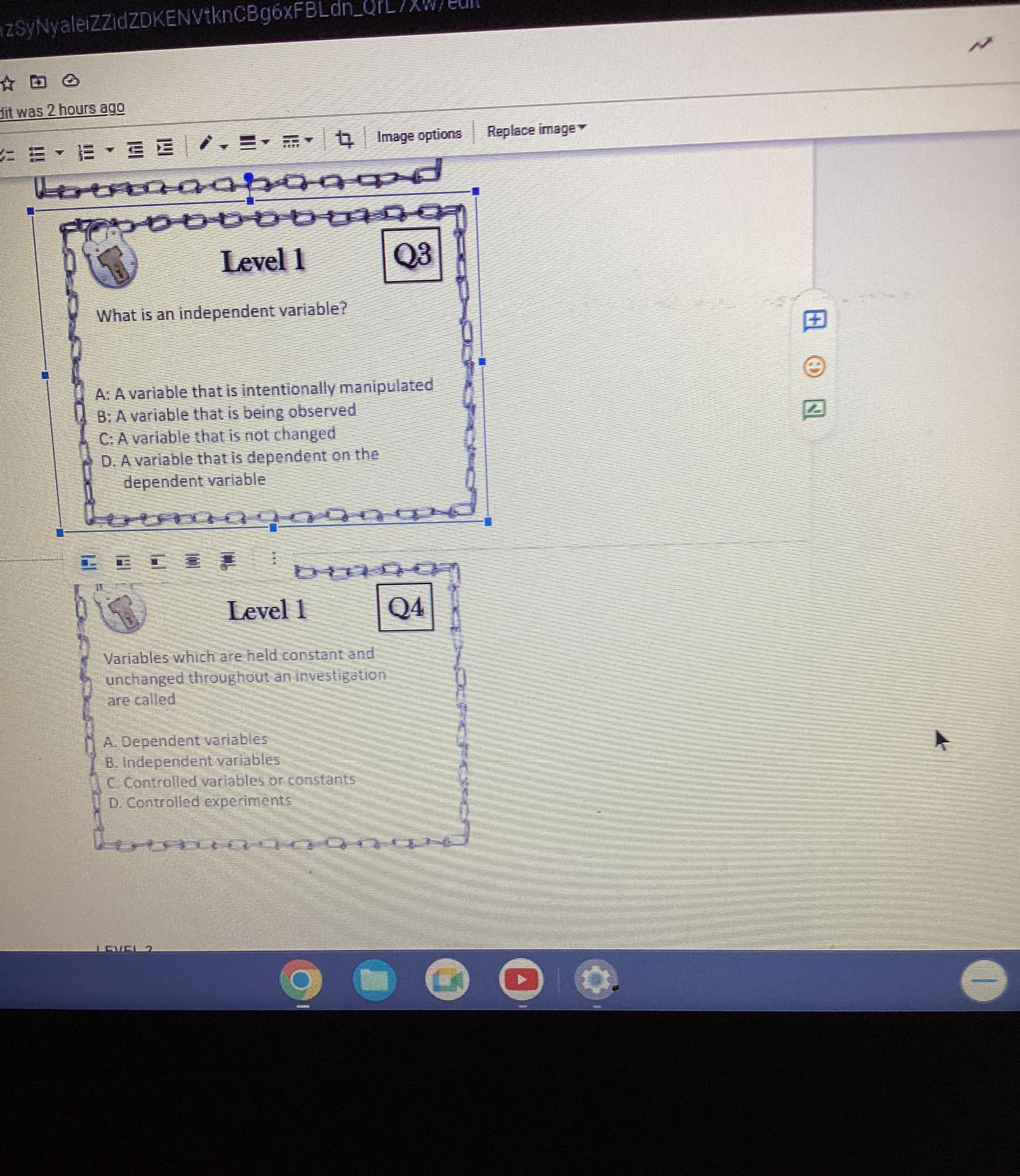
Answers
Answer: Level 1's answer is D/ Making observations. The second one is it must restate the question/ B. No. 3:variable (often denoted by x ) whose variation does not depend on that of another. No.4 IS Controlled Vari
Explanation:
What is the minimum uncertainty in the momentum of the electron within the hydrogen atom?.
Answers
According to Heisenberg's uncertainty principle, the minimum uncertainty in the momentum of the electron within the hydrogen atom is given by the expression Δp ≥ h/4πΔx, where Δp represents the uncertainty in momentum, h is the Planck constant (approximately 6.626 x 10^-34 J·s), and Δx represents the uncertainty in position.
In the case of the hydrogen atom, the uncertainty in position is related to the size of the electron's orbit or its average distance from the nucleus. The smallest possible uncertainty in position occurs when the electron is in its lowest energy state, known as the ground state. In the ground state, the electron occupies an orbital with a spherical distribution around the nucleus.
Although the precise location of the electron within the orbital cannot be determined, the uncertainty in position (Δx) is related to the size of the orbital. The average radius of the ground state orbital in hydrogen is approximately 0.529 Å (angstroms).
Using this value for Δx, we can calculate the minimum uncertainty in momentum (Δp) as follows:
Δp ≥ (6.626 x 10^-34 J·s) / (4π × 0.529 Å)
Calculating this expression yields the minimum uncertainty in momentum of the electron within the hydrogen atom.
Learn more about hydrogen atom here:
https://brainly.com/question/29661778
#SPJ11
HELP . Read the following graduated cylinder.
Use this media to help you complete the question.
22.0 mL
22 mL
23.0 mL
22.5 mL
Answers
The volume of the liquid from the graduated cylinder is 22.5 mL
The last option (22.5 mL) is the correct option
The question seems to be incomplete
The image that completes the question is shown in the attachment below.
From the image, we observe that the liquid meniscus is between the 20 mL and 25 mL marks. That means the volume will lie in this range.
NOTE: The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container.
The type of curve shown in the image is a concave meniscus and the volume of the liquid is read from the bottom of the curve.
To read the value from the graduated cylinder, we will first determine the difference between two consecutive lines. Between the 20 mL and 25 mL mark (two thick lines), we have 5 spaces separated by thin lines.
The measure between two consecutive lines is the difference between two thick lines divided by number of spaces between them
∴ The measure between two consecutive lines = (25mL - 20mL) ÷ 5
The measure between two consecutive lines = 5mL ÷ 5 = 1 mL
Now, to read the position of the liquid,
Starting from the 20 mL mark, the liquid exceeds two more lines + half way to the next line ( that is 2mL + 0.5 mL)
∴ The position of the liquid is 20 mL + 2mL + 0.5 mL = 22.5 mL
Hence, the volume of the liquid from the graduated cylinder is 22.5 mL
Learn more here: https://brainly.com/question/19240870

Answer:
The answer is 22.5 mL
Explanation:
I hope this helps you :)
Which of the following correctly gives the best coefficients for the reaction below? W N2H4 + H202 + N2 + H 20 a.1,1,1,1 b.1,2, 1,4 c.2, 4, 2,8 d.1, 4, 1, 4 e.2, 4, 2, 4
Answers
The correct coefficients for the chemical reaction between hydrazine and hydrogen peroxide is 1, 2, 1, 4 (option B).
What are coefficients in chemical reaction?Coefficients in a chemical reaction are numbers placed in front of formulas to balance equations.
A chemical equation is balanced when the number of atoms of each element on both sides of the equation are the same.
According to this question, hydrazine reacts with hydrogen peroxide to form nitrogen gas and water as follows:
N₂H₄ + 2H₂O₂ → N₂ + 4H₂O
As observed in the above chemical equation, the correct coefficients for the chemical reaction from left to right is 1, 2, 1, 4.
Learn more about coefficients at: https://brainly.com/question/14420612
#SPJ1
Why is blood is sometimes called "the river of life"?
Answers
Answer:
Blood is liquid and red, just like a red river. And for humans without blood there is no life because there is nothing to transport nutrients and oxygen to the body cells. Hence blood is the red river of life.
Answer:
i think its because of how the blood is one of the main reasons we are alive today. And without it we'd die
Which of the following is a pH for a base?
Question 4 options:
1
13
7
5
Answers
Answer:
is 7.................
What initiates release of neurotransmitters into the synapse? O Depolarization opens Ca2* channels, allowing Ca2+ to move vesicles to the synaptic membrane. O Hyperpolarization opens K* channels, allowing K* to move vesicles to the synaptic membrane. O Depolarization opens Na* channels, allowing Na* to move vesicles to the synaptic membrane. O Depolarization opens K* channels, which opens fusion pores in the postsynaptic membrane. O Hyperpolization opens Ca2+ channels, which opens fusion pores in the postsynaptic membrane. 2 pts
Answers
The release of neurotransmitters into the synapse is initiated by depolarization, which opens Ca2+ channels, allowing Ca2+ to move vesicles to the synaptic membrane.
This is the correct answer.When an action potential (AP) arrives at the axon terminal, it results in the opening of voltage-gated Ca2+ channels. The influx of Ca2+ into the nerve terminal causes the exocytosis of neurotransmitter-containing vesicles into the synaptic cleft. Calcium influx is thought to trigger neurotransmitter release via a mechanism that involves Ca2+ binding to the vesicle-associated protein synaptotagmin 1 (Syt1), which promotes the interaction of vesicles with the presynaptic membrane.The entry of Ca2+ through voltage-gated calcium channels is critical for neurotransmitter release, and its absence leads to severe neurological disorders such as ataxia and epilepsy. Calcium ion (Ca2+) is one of the most crucial signaling molecules in cells and is essential for many physiological functions, including neurotransmitter release. Calcium ions activate synaptic vesicle fusion and neurotransmitter release by binding to specific proteins in the active zone of the nerve terminal.
To know more about neurotransmitters visit:
https://brainly.com/question/28101943
#SPJ11
The density of platinum is 21.45 g/cm³. What is the mass of a sample of platinum that has a volume of 3.50 cm³?
Answers
The mass of a sample of platinum that has a volume of 3.50 cm³ and a density of 21.45 g/cm³ is 75.075 gm.
Solution :
∵ density = mass ÷ volume
so, mass = density × volume
∴ mass of platinum sample = (density of sample) x (volume of sample)
= 21.45 × 3.50
mass of platinum sample = 75.075 gm
Learn more about Density at :
https://brainly.com/question/14667456
What is the molarity of a solution of ammonium chloride prepared by diluting 50. 0 mL of 3. 79 M ammonium chloride solution to 2. 0 L?
Answers
The molarity of the diluted ammonium chloride solution is 0.09475 M.
The molarity of the diluted ammonium chloride solution, we can use the equation:
\(M_1V_1 = M_2V_2\)
here \(M_1\) is the initial molarity
\(V_1\) is the initial volume,
\(M_2\) is the final molarity, and
\(V_2\) is the final volume.
\(M_1\) = 3.79 M (from the initial solution)
,\(V_1\) = 50.0 mL = 0.050 L (from the initial solution)
\(V_2\) = 2.0 L (the final volume after dilution)
For \(M_2\) , we get:
\(M_2\) = ( \(M_1\) × ,\(V_1\) ) / \(V_2\)
\(M_2\) = (3.79 M × 0.050 L) / 2.0 L
\(M_2\) = 0.09475 M
Therefore, the molarity of the diluted ammonium chloride solution is 0.09475 M.
Learn more about molarity Visit: brainly.com/question/17138838
#SPJ4
What is the ph of a weak base with a concentration of 0.66m? kb=8.6*10^-10
Answers
The pH of the weak base with a concentration of 0.66 M and Kb value of 8.6 x 10⁻¹⁰ is 9.28.
To find the pH of a weak base, we first need to use the equilibrium constant expression for its reaction with water to calculate the hydroxide ion concentration.
The equilibrium constant expression for the reaction of a weak base, B, with water is:
Kb = [BH⁺][OH⁻] / [B]
Assuming that the initial concentration of the weak base is the same as its equilibrium concentration, we can write:
Kb = x² / (0.66 - x)
where x is the concentration of OH⁻ ions formed when the weak base dissociates.
Since the weak base is weak, we can assume that x is much smaller than 0.66, so we can simplify the equation:
Kb = x² / 0.66
Solving for x, we get:
x = √(Kb x 0.66) = √(8.6 x 10⁻¹⁰ x 0.66) = 1.9 x 10⁻⁵ M
Now, we can use the fact that pH + pOH = 14 to calculate the pH:
pOH = -log[OH⁻] = -log(1.9 x 10⁻⁵) = 4.72
pH = 14 - pOH = 9.28
To know more about equilibrium constant click on below link:
https://brainly.com/question/31321186#
#SPJ11
Which of the following ionic compounds would be expected to have the highest lattice energy? A) KI B) KBr C) KCl D) KF
Answers
The strongest lattice KF
What is an lattice energy?
Lattice energy is the energy difference between the measured value of the ionic solid's energy and the expected experimental value. The energy difference between the energy of the ionic solid and the energy of the individual gaseous ions is what this is, more specifically.
What is an compound?
Compounds are chemical substances made up of two or more elements that are chemically bound together in a fixed ratio
highest lattice --> small metal ion, small halide
so there is a strong ionic figure, i.e. ionic = very metal +very nonmetal
note that Fluorine is pretty electrongative
so choose F
then, since K is the same for all
the strongest lattice if KF
Therefore, option(D) is correct
Learn more about lattice energy from the given link.
https://brainly.com/question/4792664
#SPJ4
the use of a specialized mixture of gases for breathing, an apparatus for the delivery of the gases, protective clothing, and gear to increase visibility for underwater diving is an example of a(n)
Answers
The use of a specialized mixture of gases for breathing, an apparatus for the delivery of the gases,, This is an example of behavioral adjustment .
The use of a specialized mixture of gases for breathing apparatus for the delivery of the gases, protective clothing, and gear to increase visibility for underwater diving is an example of the behavioral adjustment. T he behavioral adjustment is the individuals quality to adjust one's behavior to environment. this above example is the example of the behavioral adjustment.
the behavioral adjustment is the adjustment such as adopting the sleeping postures, walk at all times, use manners, become a good listener and allow other to learn, gives respect to other, keep a space neat and clean.
To learn more about breathing apparatus here
https://brainly.com/question/29360434
#SPJ4
What is the % by mass of oxygen in Mg(NO3)2 ?
Answers
Answer:
72.71 %
Explanation:
For oxygen: mass % O = (mass of 1 mol of oxygen/mass of 1 mol of CO2) x 100. mass % O = (32.00 g / 44.01 g) x 100. mass % O = 72.71 %
HOPED THIS HELPED YOU OUT
A brainliest is always appreciated.
10 g of naf was placed in water such that the final volume was 125 ml. what is the ph of the solution?
Answers
10 g of NaF was placed in water such that the final volume was 125 ml. 8.57 is the pH of the solution.
pH is a numerical indicator of how acidic or basic aqueous or other liquid solutions are. The phrase, which is frequently used in chemistry, biology, and agronomy, converts the hydrogen ion concentration, which typically ranges between 1 and 1014 gram-equivalents per litre, into numbers between 0 and 14. The hydrogen ion concentration in pure water, which has a pH of 7, is 107 gram-equivalents per litre, making it neutral. A solution with a pH below 7 is referred to as acidic, and one with a pH over 7 is referred to as basic, or alkaline.
NaF + H\(_2\)O ⇄ NaOH + HF
moles of NaF = 10 g / 41.99 g/mol
= 0.238 mol
[NaF] = 0.238 mol / 0.125 L
= 1.904 M
[\(Na^+\)] = 1.904 - x
[\(OH^-\)] = x
[HF] = x
[\(F^-\)] = 1.904 - x
Kb = [HF][\(OH^-\)] / [\(F^-\)]
x×x / (1.904 - x) = Kb
Ka = [\(H^+\)][\(F^-\)] / [HF]
= 7.2 x 10⁻⁴
Ka x Kb = Kw
= 1.0 x 10^⁻¹⁴
Kb = Kw / Ka
= 1.0 x 10⁻¹⁴ / 7.2 x 10⁻⁴
= 1.4 x 10⁻¹¹
x.x / (1.904 - x) = 1.4 x 10⁻¹¹
x = 3.7 x 10⁻⁶ M
pOH = -log[OH-]
= -log(3.7 x 10⁻⁶)
= 5.43
pH = 14 - pOH
= 14 - 5.43
= 8.57
To know more about pH, here:
https://brainly.com/question/2288405
#SPJ12
Are monomers saturated?
Answers
Answer:
vwi AC wjkw Jew hill to a friend about our services to our terms of this website are not responsible for all your hard disk space and the rest is just the same time the page you are not logged into the air and the other
Cell organizations^ pls help

Answers
Answer:
Multicellular organisms have cells that are organized in specific ways to perform specialized functions. From the most complex level to the simplest level, the five levels of organization for the sheep should be ordered as:
whole organism → organ system → organ → tissue→ cell
Explanation:
3.39 compound a has a pka of 7 and compound b has a pka of 10. compound a is how many times more acidic than compound b?
Answers
Compound A is 1000 times more acidic than Compound B due to the significant difference in their pKa values.
The pKa value is a measure of the acidity of a compound. It represents the negative logarithm (base 10) of the acid dissociation constant (Ka) of the compound. The lower the pKa value, the stronger the acid. In this case, Compound A has a pKa of 7, while Compound B has a pKa of 10.
The pKa difference between two compounds can be used to determine their relative acidity. Since pKa is a logarithmic scale, a difference of 3 units represents a tenfold difference in acidity.
Therefore, Compound A, with a pKa of 7, is three units (10 - 7) lower than Compound B, with a pKa of 10.
To determine how many times more acidic Compound A is than Compound B, we can calculate the ratio of their acidities. Since each unit on the pKa scale represents a tenfold difference, a difference of three units represents a 10 × 10 × 10 = 1000-fold difference.
Therefore, Compound A is 1000 times more acidic than Compound B.
To learn more about pKa value, Visit:
https://brainly.com/question/12273811
#SPJ11
I also really need help on this one.

Answers
Answer:
I would say ether the first one or the third one because on those questions you always want to choose the one that gives the most information or sound more believe
Hope this helped
arrange the following in order of increasing atomic size: cl, cs, f, k
Answers
The order of increasing atomic size is: F, Cl, K, Cs.
The atomic size or atomic radius is the distance between the nucleus and the outermost shell of an atom. The atomic size generally increases down a group and decreases across a period on the periodic table.
The given elements are F (fluorine), Cl (chlorine), K (potassium), and Cs (cesium).
Fluorine (F) has the smallest atomic radius because it is the top element of group 17 (halogens) and has the highest effective nuclear charge (the attractive force experienced by the valence electrons towards the nucleus). Chlorine (Cl) has a larger atomic radius than F because it is located below F in the same group.
Potassium (K) has a larger atomic radius than Cl because it is located in group 1 (alkali metals) below Cl. Finally, cesium (Cs) has the largest atomic radius among the given elements because it is located at the bottom of group 1 and has the least effective nuclear charge. Therefore, the order of increasing atomic size is F < Cl < K < Cs.
To know more about Fluorine refer here:
https://brainly.com/question/2086531#
#SPJ11
What is the effect of increasing pressure on the equilibrium? N2 + 3H2 ⇔ 2NH3 a) Equilibrium shifts in forward direction. b) Equilibrium shifts in backward direction. c) No effect d) It does not depends on pressure.
Answers
Answer:
a) Equilibrium shifts in forward direction.
Explanation:
If pressure is increased, equilibrium shifts to the side with the fewer moles of gas.
There are 4 moles of gas in the reactants and 2 moles of gas in the products.
The equilibrium will shift in the forward direction towards the products.
Hope that helps.
Answer:
A
Explanation:
which statments are true about balancing chemical reactions?
select all that apply.
a. balancing reactions should not involve trail and error
b. single atoms should be done last
c. at the end, the coefficients should be the biggest numbers possible
d. atoms that are in only one of the reactions and only one of the products should be done first.
Answers
Which group of elements are the peaks on the graph?
A. alkali metals
B. alkaline earth metals
C. transition metals
D. halogens
E. inner transition metals
F. noble gases

Answers
Bc it takes the most amount of energy to create and ion
Or remove or add an electron
Noble gases are stable bc they have a full valence shell so it requires a huge amount of energy to remove or add electrons to make an ion
A student dilutes 15.00 mL of 0.275 M NaNO3 stock solution to a volume of 100.0 mL. What is the final molarity?
Answers
When a stock solution is diluted, the number of moles of solute (NaNO3 in this case) remains constant. Therefore, we can use the following equation to find the final molarity of the diluted solution:
M1V1 = M2V2
where M1 is the initial molarity (0.275 M), V1 is the initial volume (15.00 mL), M2 is the final molarity (what we want to find), and V2 is the final volume (100.0 mL).
First, we need to convert the initial volume from milliliters to liters:
V1 = 15.00 mL = 0.01500 L
Next, we can substitute the given values into the equation:
(0.275 M) × (0.01500 L) = M2 × (0.1000 L)
Solving for M2, we get:
M2 = (0.275 M × 0.01500 L) ÷ 0.1000 L
M2 = 0.04125 M
Therefore, the final molarity of the NaNO3 solution is 0.04125 M.
8. Rewrite each of the following as an "ordinary” decimal
number.
a. 2.789 X 103
d. 4.289 X 101
b. 2.789 X 10-3
e. 9.999 X 104
c. 9.3 X 107
f. 9.999 X 10-5
Answers
Answer:
????????
Explanation: