What is the brown liquid for that the grasshopper may “spit” out?
Answers
Answer:
It is called "tobacco juice"
Explanation:
When a grasshopper is picked up they spit the brownish liquid known as "tobacco juice." They use it as some sort of defense mechanism to protect them from attacks by predators.
Hope this helps:)...if not then sorry for wasting your time and may God bless you:)
Related Questions
Which shows that friction is undesirable?
a. Pushing furniture
b. Lighting a matchstick
c. Walking on wet floors
d. Approaching a spotlight
Answers
C. Walking on wet floors shows that Friction is undesirable
what is the apparatus used to carry out paper chromatography?
Answers
Answer:
the apparatus are listed below
Explanation:
The apparatus used to carry out paper chromatography are:
• Chromatography paper
• Solvent (or water)
• Pencil
• Ink (pen)
• Beaker
The apparatus used to carry out the paper chromatography are chromatography paper ,organic solvents and glass capillary.
What is paper chromatography?Paper chromatography is an analytical method used to separate colored chemicals or substances.
Paper chromatography is a quick and easy process to conduct. Most of the time, it is used to test the purity of compounds and to identify substances.
It is primarily used as a teaching tool, having been replaced by other chromatography methods, such as thin-layer chromatography.
Listed are the apparatus used to carry out the paper chromatography-
Chromatography paper Organic solvents, acids, bases, buffers, etc. to make mobile phase. A glass container with a cap. Something hold the chromatography paper: a stick, or a hook. A piece of glass capillary to load the sample onto the paper.Learn more about paper chromatography ,here:
https://brainly.com/question/14171744
#SPJ2
Why does a solid fill only part of a closed jar while the same mass of a gas fills the whole jar?
Answers
Answer:
because of atom don't put that as an answer
Explanation:
i need point to do finals
The different behavior of particles in solids and gases, including the intermolecular forces and kinetic energy, leads to solids filling only part of a closed jar while the same mass of a gas fills the whole jar. A solid fills only part of a closed jar, while the same mass of a gas fills the whole jar due to the differences in the behavior of particles in each state of matter.
1. Solid: In a solid, the particles are tightly packed and held in a fixed position. They have a definite shape and volume. The intermolecular forces between the particles are strong, preventing them from moving freely. As a result, solids occupy a specific amount of space within a container.
2. Gas: In a gas, the particles are spread out and move freely. They have no definite shape or volume. The intermolecular forces between the particles are weak, allowing them to move and fill the available space. As a result, gases can expand to fill the entire volume of a container.
When the same mass of a gas and a solid are placed in a closed jar, the gas particles have higher kinetic energy and are more mobile. They collide with each other and the walls of the container, exerting pressure and spreading out to occupy the entire space. Since the intermolecular forces are weak, the gas particles are not constrained by a fixed arrangement.
Learn more about Intermolecular Forces here:
https://brainly.com/question/12243368
#SPJ3
the unknown aldehydes/ketones and sodium borohydride are all more soluble in ethyl acetate than methanol. would ethyl acetate therefore be a more suitable solvent for this experiment? why or why not? provide a reaction scheme to help explain your answer:
Answers
Ether acetate would be a suitable solution for the experiment based on the solubility of sodium borohydride and the mysterious aldehydes/ketones.
It is necessary to decrease unidentified aldehydes/ketones with sodium borohydride while submerging the reactants and products.
An unnamed ketone or aldehyde is reduced using sodium borohydride. The resulting alcohol (RCH2OH/R2CHOH), sodium borate (NaBO2), and hydrogen gas are produced when the unknown aldehyde or ketone (RCHO/R2CO) reacts with sodium borohydride (NaBH4). (H2).
Ethyl acetate would be a better solvent overall for the experiment because it would make the reactants and products more soluble.
Learn more about Ether acetate:
https://brainly.com/question/30434200
#SPJ4
A chemist is identifying the elements present in a sample of seawater. What characteristic of an element's atoms always determines the element's identity?
Group of answer choices (Could someone help me? I am kind of confused) Thank you so much
A. The number of valence electrons
B. The location of valence electrons
C. The number of neutrons
D. The number of protons
Answers
HELP!! WILL GIVE 100 points.
Which term describes this molecular shape?
A. Tetrahedral
B. Linear
C. Trigonal Planar
D. Bent

Answers
Answer:
C. trigonal Planar
Explanation:
Tetrahedral is 4 connected
Bent is 2 connected with a lone pair
Linear is 2 connected in a line
Trigonal Planar is the one with 3 connected.
Answer:
C: Trigonal Planar
Explanation:
Tetrahedral is four connected
Bent is 2 linked with a lone pair
Linear is two connected in a line
Trigonal Planar is the one with 3 connected.
When a new substance has formed, has a physical or chemical) change occurred?
(4 Points)
A) chemical
B) physical
Answers
Answer: A) Chemical Change
Explanation:
A chemical change create a NEW type of matter that CANNOT be reversed!
Example: Spolied milk
Example: Green tomatoe turns red
Hope it’s helpful! :)
a mixture of ethanol and ethyl acetate is heated in a closed system at 100 kpa to a temperature of 74°c, and two phases are observed to be present. what are the possible compositions of the liquid and vapor phases?
Answers
The possible compositions of the liquid phases are: \(x_{1} =0.15\) and \(x_{2} =0.79\), while those of vapor phases are: \(y_{1} =0.2\) and \(y_{2} =0.7\)
The given binary mixture of ethanol and ethyl acetate at 100 kPa.
Given plot is temperature versus mole fraction (T-xy) at constant pressure 100 kPa.
In the given plot, the upper curve represents the dewpoint curve and lower curve represents bubble point curve.
At a given temperature 74 C, we can determine the mole fractions as follows:
1. We draw a horizontal lie from 74 °C mole fraction 0 to 1 and it touches lower curve at two points and upper curve at two points.
2. We also draw a vertical line from points where it touches the lower curve gives the composition of liquid and draw a vertical line from points where it touches the upper curve gives the vapor compositions.
From the plot given below, the compositions of liquid where the vertical line touches lower curve are: \(x_{1} =0.15\) and \(x_{2} =0.79\)
And those for vapor phases where the line touches the upper curve are:
\(y_{1} =0.2\) and \(y_{2} =0.7\)
Learn more about the chemical mixtures here:
https://brainly.com/question/17975594
#SPJ4

400 ml of a 75 M solution of H2SO4 is needed to for a lab. The stock solution is 16.0 M. Calculate how much stock is needed to make the solution.
Answers
Answer:
The volume of stock solution needed to make the solution is 1875 ml
Explanation:
The parameters given are;
The volume of 75 M solution of H₂SO₄ = 400 ml
The concentration of stock solution = 16.0 M
Number moles per liter of stock solution = 16 moles
Number of moles in required 400 ml solution = 0.4×75 = 30 M
Volume of stock solution that contains 30 M = 30/16×1 = 1.875 l
The volume of stock solution that is required = 1875 ml
If two elements have the same number of protons but different number of neutrons, can they be atoms of the same elements?
Answers
If two elements have the same number of protons but different number of neutrons, can they be atoms of the same elements are called isotopes.
Atoms of the identical detail that incorporate the identical variety of protons, however special numbers of neutrons, are referred to as isotopes. Isotopes of any given detail all incorporate the identical variety of protons, in order that they have the identical atomic size (for example, the atomic variety of helium is usually 2). An isotope is one in every of or greater kinds of the identical chemical detail. Different isotopes of an detail have the identical size of protons withinside the nucleus, giving them the identical atomic size however a special variety of neutrons giving every elemental isotope a special atomic weight.
Thus, that species are known as isotopes.
To learn more about isotopes check the link below:
https://brainly.com/question/12694278
#SPJ4
the smallest representative unit of an ionic compound is called a ?
Answers
The lowest representational unit for an ionic compound is a formula unit.
What does a chemical representative unit mean?The smallest unit in which a substance naturally exists is called a representative particle. The atom is the representative particle for most elements. The atom is the representative particle for most elements. Iron, carbon, and helium are made up of individual iron, carbon, and helium atoms.
What are atoms smaller than?Subatomic is defined as "less than an atom." Protons, neutrons, and electrons make up atoms. Even smaller particles known as quarks are the building blocks of protons and neutrons. Physicists believe quarks are elementary particles based on the evidence that is currently available.
To learn more about ionic compound visit:
brainly.com/question/29005103
#SPJ4
which of the following is a valid mole ratio from the balanced equation 2fe2o3 3c → 4fe 3co2?
Answers
The valid mole ratio is: 2 moles Fe2O3 : 3 moles C.
The balanced chemical equation is 2Fe2O3 + 3C → 4Fe + 3CO2.
This chemical equation represents the reaction of Fe2O3 with C, producing Fe and CO2.Mole ratio is the ratio of moles of one substance to another substance in a chemical equation.
To determine the valid mole ratio, we use the coefficients in the balanced chemical equation. These coefficients represent the number of moles of each substance present in the reaction
.For the given balanced chemical equation 2Fe2O3 + 3C → 4Fe + 3CO2, there are several possible mole ratios. We can choose any two substances, but the ratio of moles must be the same for all substances.
So, let's find some valid mole ratios:
2 moles Fe2O3 : 3 moles C2 moles Fe2O3 : 4 moles Fe3 moles C : 3 moles CO24 moles Fe : 3 moles CO22 moles Fe : 3 moles C. We can simplify these mole ratios by dividing all the coefficients by the smallest coefficient in each ratio.
For example, for the ratio 2 moles Fe2O3 : 3 moles C, the smallest coefficient is 2, so we divide all the coefficients by 2:1 mole Fe2O3 : 1.5 moles C
Now, we can choose the valid mole ratio from the given balanced equation, which is 2 moles Fe2O3 : 3 moles C.
Therefore, the answer is: 2 moles Fe2O3 : 3 moles C.
Learn more about mole at: https://brainly.com/question/29367909
#SPJ11
The redox carriers that comprise most of the electron transport chain and are responsible for accepting and donating electrons are:
Answers
The redox carriers that comprise most of the electron transport chain and are responsible for accepting and donating electrons are Ubiquinone , Cytochrome , Iron-sulfur proteins , Flavoproteins .
1. Ubiquinone (also known as coenzyme Q) - it is a small, lipid-soluble molecule that shuttles electrons between Complexes I, II, and III in the inner mitochondrial membrane.
2. Cytochrome c - it is a small, water-soluble protein that carries electrons between Complex III and Complex IV in the inner mitochondrial membrane.
3. Iron-sulfur proteins - they are a group of proteins that contain clusters of iron and sulfur atoms that act as electron carriers in Complexes I, II, and III.
4. Flavoproteins - they are a group of proteins that contain a flavin molecule that accepts and donates electrons in Complexes I and II.
These redox carriers work together to transfer electrons from NADH and FADH2 to molecular oxygen, generating a proton gradient across the inner mitochondrial membrane that drives ATP synthesis.
To learn more about redox refer here:
https://brainly.com/question/15678074#
#SPJ11
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols
. strip of solid iron metal is put into a beaker of 0.072M Cu(NO3)2 solution.
Answers
This equation represents the solid iron (Fe) reacting with the aqueous copper(II) nitrate (Cu(NO3)2) solution to produce aqueous iron(II) nitrate (Fe(NO3)2) and solid copper (Cu).
In this situation, a chemical reaction does occur between iron (Fe) and copper(II) nitrate (Cu(NO3)2). The iron reacts with the copper(II) ions in the solution to form iron(II) ions and copper metal.
The balanced chemical equation for the reaction is:
Fe(s) + Cu(NO3)2(aq) → Fe(NO3)2(aq) + Cu(s)
This equation represents the solid iron (Fe) reacting with the aqueous copper(II) nitrate (Cu(NO3)2) solution to produce aqueous iron(II) nitrate (Fe(NO3)2) and solid copper (Cu).
Learn more about nitrate here:
https://brainly.com/question/32128844
#SPJ11
What is the area on the earth's surface directly above where an earthquake originates?
Answers
Answer:
The epicenter
If Acetanilide, Aniline, and Anisole undergo bromination reaction, how would you arrange the three substituent groups (acetamido, amino, and methoxy) in order of decreasing ability to activate the benzene ring?
Answers
If Acetanilide, Aniline, and Anisole undergo bromination reaction, then we can arrange the three substituent groups (acetamido, amino, and methoxy) in order of decreasing ability to activate the benzene ring would be: Aniline (amino)> Acetanilide(acetamido)> Anisole(methoxy).
Bromination reaction in organic chemistry is electrophilic aromatic substitution, which involves the substitution of a hydrogen atom on an aromatic ring with a bromine atom.
If the substituent on the aromatic ring is amino group the name for this benzene derivative is aniline. The amino group (-NH₂) is a strong activator of the benzene ring. It donates electrons through resonance, increasing the electron density on the ring and making it more reactive towards electrophilic substitution reactions such as bromination.
If the substituent on the aromatic ring is acetamido group the name for this benzene derivative is acetanilide. The acetamido group (-NHCOCH₃) is a moderate activator of the benzene ring. Although it is not as strong as the amino group, it still donates electrons through resonance, leading to increased reactivity in bromination reactions compared to the absence of any substituent.
If the substituent on the aromatic ring is methoxy group the name for this benzene derivative is anisole. The methoxy group (-OCH₃) is a weak activator of the benzene ring. It donates electrons through the inductive effect, which is a less efficient electron-donating mechanism compared to resonance. Consequently, the activating effect of the methoxy group is weaker than that of the amino and acetamido groups.
Therefore, the order of decreasing ability to activate the benzene ring in bromination reactions would be: Aniline(amino)>Acetanilide(acetamido)> Anisole(methoxy).
To know more about bromination reaction here
https://brainly.com/question/31425357
#SPJ4
Choose an example of a reaction to which Markovnikov's rule applies.
O CH₂=CH-CH2-CH3 + HBr CH₂ Br=CH2-CH2-CH3
O CH,=CH-CH, CH3 + HBr → CHg =CHBr–CH2–CH3
O CH,=CH-CH,—CH, + HBr → CH,Br–CHBr–CH2–CH, + HBr CH₂Br-CH2-CH2-CH3
O CH₂=CH-CH2-CH3 O CH,=CH-CH2–CH3 + HBr → CH3–CHBr–CH2–CH3
Answers
The example of a reaction to which Markovnikov's rule applies is: CH₂=CH-CH₂-CH₃ + HBr → CH₂Br-CH₂-CH₂-CH₃
In this reaction, the hydrogen atom from HBr adds to the carbon atom with the fewer alkyl substituents (less substituted carbon), while the bromine atom adds to the carbon atom with more alkyl substituents (more substituted carbon). This follows Markovnikov's rule, which states that in the addition of a protic acid (such as HBr) to an asymmetrically substituted alkene, the hydrogen atom adds to the less substituted carbon and the other atom adds to the more substituted carbon.
To learn more about Markovnikov's, https://brainly.com/question/32087294
#SPJ11
Rubidium, Rb, has a heat of vaporization, Hvap of 69.0 kJ/mol and an entropy of vaporization, Svap of 71.9 J/K mol. Calculate the normal boiling point of rubidium. Group of answer choices
Answers
nswer: 959.67 K or 686.67 C
Explanation:
Entropy of Vaporization (S) = Heat of Vaporization (H) / T ( boiling point) (in Kelvin)
1.) Flip the equation to have T as the product --> T = H/S
2.) We need to change H from kJ to J --> 69.0 kJ/mol --> 69000J/mol
3.) Substitute numbers in the equation --> (69000 kJ/mol) / (71.9 J/Kmol) = T
4.) T = 959.67 K or in Celcius --> 959.67 - 273 = 686.67 C
I need help with this question I can’t find the answer to it

Answers
113.89/3
=
37.96333
Research the compositions of Pennies. What was the composition of each of your Pennies prior to treatment
Answers
Answer:
History of composition
Years Material Weight (grains)
1944–1946 gilding metal (95% copper, 5% zinc) 48 grains
1947–1962 bronze (95% copper, 5% tin and zinc) 48 grains
1962 – September 1982 gilding metal (95% copper, 5% zinc) 48 grains
October 1982 – present copper-plated zinc (97.5% zinc, 2.5% copper) 38.6 grains
N204(0) + 2NO2(g)
Colorless Brown
Keq = 6.16 x 103
What is the predicted direction of change?
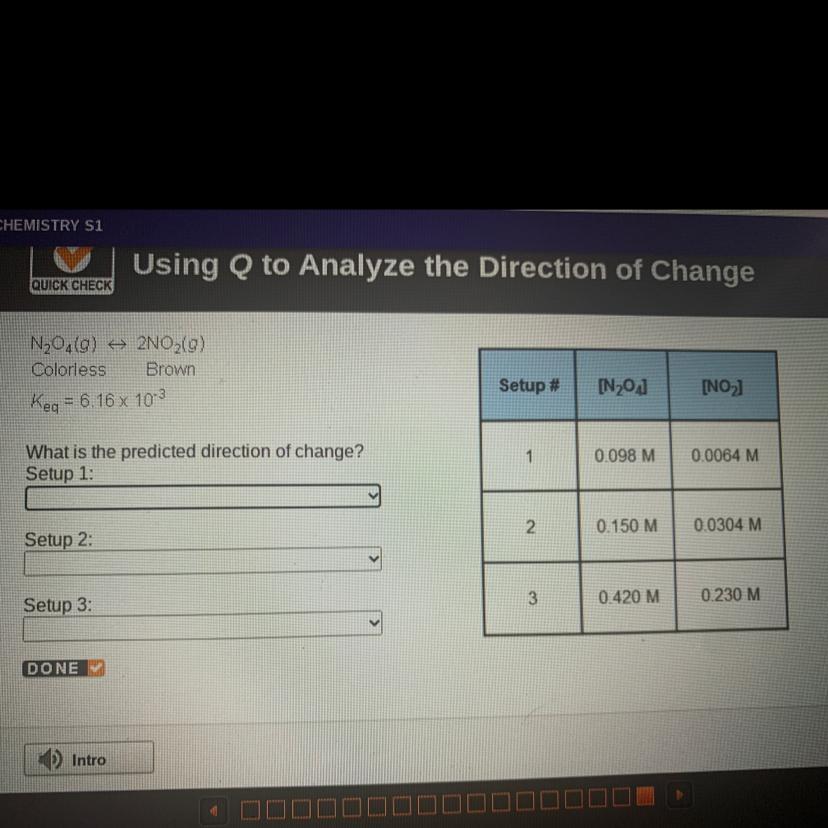
Answers
setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanationThe reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
\(\tt Q=\dfrac{C]^c[D]^d}{[A]^a[B]^b}\)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
\(\tt Q=\dfrac{[NO_2]^2}{[N_2O_4]}\)
Setup 1 :
\(\tt Q=\dfrac{0.0064^2}{0.098}=0.000418=4.18\times 10^{-4}\)
Q<K⇒The reaction moved to the right (products)
Setup 2 :
\(\tt Q=\dfrac{0.0304^2}{0.15}=0.00616=6.16\times 10^{-3}\)
Q=K⇒the system at equilibrium
Setup 3 :
\(\tt Q=\dfrac{0.230^2}{0.420}=0.126\)
Q>K⇒The reaction moved to the left (reactants)
Answer:
The system will shift toward the products
The system is at equilibrium
The system will shift toward the reactants
Explanation:
This is correct on edg... Good Luck!!!!
Which rule concerning oxidation numbers is true?
A. When a redox reaction occurs, there will always be at least one atom that is oxidized and at least one atom that is reduced.
B. Oxidation is the process by which an atom loses electrons.
C. Reduction is the process by which an atom gains electrons.
D. All of the Above
E. None of the Above
Answers
Answer:
B
Explanation:
because an atom is the smallest particles that have electrons, neutron and protons
Plutonium-238 is a radioactive element used as a power source in spacecraft like Voyager and New Horizons. It has a half life of 87.7 years. Suppose we have 2 kg of plutonium-238 right now. How much plutonium will be left in 87.7 years? A) None B) 0.25 kg C) 0.5 kg D) 1.0 kg E) 2 kg
Answers
The answer is C) 0.5 kg. This is because Plutonium-238 has a half-life of 87.7 years, which means that after 87.7 years, half of the original amount of Plutonium-238 will remain. In this case, that would be 2 kg * 0.5 = 0.5 kg.
Plutonium-238 is a radioactive element used as a power source in spacecraft like Voyager and New Horizons. It has a half-life of 87.7 years. Suppose we have 2 kg of plutonium-238 right now. Radioactive decay is a random event. So, it is impossible to predict when a specific atom will decay. But we can find how much radioactive material is remaining after a specific period of time.
The half-life of a radioactive material is the time required for half of the radioactive material to decay. The formula to calculate the remaining material is:
N(t) = N0 × (1/2)^(t/t1/2)
Where N(t) is the remaining material at time t, N0 is the initial material, t1/2 is the half-life, and t is the elapsed time.
The initial material is 2 kg, half-life is 87.7 years, and the elapsed time is also 87.7 years.
N(87.7) = 2 kg × (1/2)^(87.7/87.7)= 1 kg × 0.5= 0.5 kg
Therefore, the amount of plutonium remaining after 87.7 years will be 0.5 kg. So, the answer is option C.
For more such questions on Plutonium , Visit:
https://brainly.com/question/13217330
#SPJ11
in 1 to 2 sentences explain why a structural formulas are good for depicting polymers
Answers
Answer:
they explain the properties and structure of the compound which empirical molecular formulars cannot explain
The structural formulas are good for depicting polymers because polymers are the chemical compounds attached to the long chain with ac other which is impossible to represent other than the structural formula.
What are structural formulas?The structural formulas are the graphic representation or diagrammatical representation of the structure of any chemical formula in organic and inorganic chemistry they are mostly used.
In Polymers, they are long chains of chemicals compound can be shown diagrammatically or graphically to understand the attachment t of the bonds within them which signifies the physical properties too.
Therefore, polymers are the chemical compounds attached to the long chain with ac other which is impossible to represent other than the structural formula which is why structural formulas are good for depicting polymers.
Learn more about structural formulas, here:
https://brainly.com/question/24418000
#SPJ2
the initial temperature of 100.0 ml of water was 23 oc. the water was then heated to 87 oc. how much heat (in j) was required for this temperature change?
Answers
The heat was required for his temperature change 26752 J.
given that :
volume : 100 mL
The initial temperature = 23 °C
The final temperature = 87 °C
ΔT = 87 °C - 23 °C = 64 °C
density = mass / volume
mass = density × volume
= 1 × 100
= 100 g
the specific heat expression is given as :
Q = m c ΔT
where,
specific heat capacity , c = 4.18 J/g °C
mass of water , m = 100 g
change in temperature , ΔT = 64 °C
Q = 100 × 4.18 × 64
Q = 26752 J
Thus, the initial temperature of 100.0 ml of water was 23 °C . the water was then heated to 87 °C .heat (in j) was required for this temperature change is 26752 J.
To learn more about specific heat here
https://brainly.com/question/21041726
#SPJ4
A donut has a density of 0.75 g/cm cubed and a mass of 100.0g. What is the volume of the donut?
Answers
Answer:
133.333333333 cm^3
Explanation:
Volume = Mass/Density
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2V2 V1/T1 = V2/T2 PV = nRT V1/T1 x V2/T2

Answers
Answer:
Option b (V1/T1 = V2/T2) is the right alternative or the new volume will be "0.048 L".
Explanation:
The given values are:
Temperature,
T₁ = 25°C
or,
= 298.15 K
T₂ = 50°C
or,
= 323.15 K
Volume,
V₁ = 45 mL
or,
= 0.045 L
V₂ = ?
As we know,
⇒ \(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Or,
⇒ \(V_2=\frac{V_1\times T_2}{T_1}\)
On substituting the values, we get
⇒ \(=\frac{0.045\times 323.15}{298.15}\)
⇒ \(=\frac{14.541}{298.15}\)
⇒ \(=0.048 \ L\)
sicl2f2 polar or nonpolar
Answers
The correct answer is Polar (:
SiCl₂f₂ is a polar compound. Polar compounds are those with distinct regions of positive and negative charge.
What do you mean by polar compound ?The polar compounds have distinct zones of positive and negative elements like nitrogen, oxygen, or sulphur, charge. Higher boiling, more aromatic, and heteroatom-containing components.
Dichlorodifluorosilane is a polar molecule. As fluorine and chlorine have higher electronegativity, they take electrons away from the silicone atom in the middle, partially changing its charge and giving the fluorine and chlorine atoms a negative charge.
The polarity and non-polarity characteristics of molecules are determined by the atoms' positions. SiCl₂f₂ is the polar molecule.
Thus, SiCl₂f₂ is a polar compound.
To learn more about the polar compound, follow the link;
https://brainly.com/question/14752836
#SPJ2
Hi! Can you help me?
Is NaCI an element, compound, or mixture?
Thanks!
Answers
Answer:
Compound
Explanation:
Sodium chloride, commonly known as salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl
A shielded nucleus will absorb ____ from a deshielded nucleus and will have a _____ chemical shift.
Answers
A shielded nucleus will have a reduced chemical shift and will absorb upfield from a deshielded nucleus.
The nucleus is the central part of an atom that contains most of its mass and all of its positive charge. It is composed of protons and neutrons, which are held together by a strong nuclear force. The number of protons in the nucleus determines the element that the atom belongs to, while the number of neutrons can vary and give rise to different isotopes of the same element. The properties of the nucleus are essential in determining the behavior of the atom in chemical and physical reactions. Nuclei can also undergo nuclear reactions such as fission and fusion, which release enormous amounts of energy and are the basis for nuclear power and nuclear weapons. The study of nuclei and their properties is known as nuclear physics and is a fundamental area of modern science.
Learn more about nucleus here:
https://brainly.com/question/9837741
#SPJ4