What is the change of state in which a gas becomes a liquid?
Answers
Answer:
Condensation
Explanation:
Answer:
Condensation
Explanation:
When a gas changes to a liquid it is known as condensation. It happens when molecules in a gas slowly reduce their movement speed.
HOPE THIS HELPED
Related Questions
How many molecules are in 48.0 grams of NaOH?
Answers
Hope this helped :)
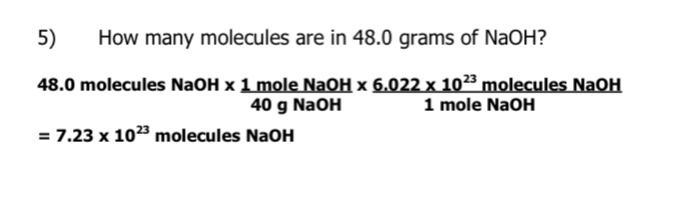
How many moles are in 8.5 x 10²⁵ molecules of Carbon Dioxide?
Answers
Answer:
141.20 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{8.5 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 141.19601...\)
We have the final answer as
141.20 molesHope this helps you
Explain the relationship between the number of valence electrons and the reactivity of a metal.
Answers
Got this from a quizlet I used a couple days ago, hope it helps!
True/False (A= True & B =False) 46. Blood is a tissue. 47. Elastic cartilage is found in the ears, nose, epiglottis and larynx. 48. If you have a neoplasm you hope it is benign. 49. All cancers are neoplasms but not all neoplasms are cancer. 50. Plasma cells produce Histamine 51. The liver is one organ that can partially regenerate because it is made of epithelial cells 52. Keratin is a waterproofing protein found in epithelial cells
Answers
Answer:
46.A 47.B 48.B 49.A.50.A.51.B.52.B
The answer of the questions serially is true, false,false ,true,true,false and false where blood is a tissue which circulated by the pumping organ that is heart.
What is heart?
Heart is a muscular organ which is present in most of the animals , it is responsible for pumping blood via the blood vessels to the entire body.The blood which is pumped carries oxygen and nutrients along with it.Along with it on it's way back to heart it carries carbon dioxide to the lungs.
Heart has an approximate size of a closed fist and is present between the lungs in the middle compartment of the chest. It is divided in to for chambers , the upper chambers called atria and lower ones called ventricles.
The wall of the heart is made up of three layers : epicardium,myocardium and endocardium. It pumps blood with a rhythm which is determined by a group of cells which are called the pacemaker cells present in the sinoatrial node.
Learn more about heart,here:
https://brainly.com/question/16566688
#SPJ5
Which of the following notations is the correct noble gas configuration for Li?
O A. 1s²2s¹
OB. 1s22s
C. [He]2s¹
D. [Hel1 s22s¹
Answers
The right option is C, the correct noble gas notations for Li is [He] 2s¹.
We know that the atomic number of Li is 3 , so it will have 3 electrons in total, in filling electrons the electronic configuration is came as follows:
1s²2s¹ , but we asked for noble gas electronic configuration , so in this type of way of writing a noble gas configuration , we write by using the most nearer electronic configuration of a noble gas.
Let us see each option in each case,
In option A, it is the normal electronic configuration with no noble gas involved so it will be wrong.In option B, it is 1s²2s , which is 2 electrons only, so it will be wrongIn option C, it is using 2s² for He and the rest one electron for Li is written as 2s¹, so it will be the right answer.In option D, if we look at total number of electrons it will be 5 electrons , so this one will also be wrong.So the correct notation will be [He]2s¹ which is option C.
To know more about electronic configuration, please refer:
https://brainly.com/question/26084288
#SPJ1
Lucite contains 59.9 g C, 8.06 g H,
and 32.0 g O. You want to determine the empirical formula.
How many moles of C are in the sample?

Answers
Number of moles of C = (Mass of C)/(Molar Mass of C)
Number of moles of C = (59.9 g)/(12.01 g/mol)
Number of moles of C = 4.98 mol
789$ Rubin by David via
(578996)
write the full ground‑state electron configuration for that element.
a. S: b. Kr :
c. Cs :
Answers
The ground‑state electron configuration of element sulfur (S) is; 1s² 2s² 2p⁶ 3s² 3p⁴, element krypton (Kr) is; 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶, and the element cesium (Ce) is; 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s¹.
The element S represents sulfur, which has an atomic number of 16. The full ground-state electron configuration for sulfur is obtained by filling up the orbitals with electrons according to the Aufbau principle and the Pauli exclusion principle.
Starting with the lowest energy level, the 1s orbital can hold a maximum of 2 electrons. Moving to the next energy level, the 2s orbital is filled with 2 electrons as well. Then, the 2p orbital is filled with a total of 6 electrons, distributed among its three sub-orbitals (2px, 2py, 2pz).
Putting it all together, the full ground-state electron configuration for sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴.
The element Kr represents krypton, which has an atomic number of 36. Similarly, we follow the Aufbau principle and the Pauli exclusion principle to determine the electron configuration.
Starting with the 1s orbital, it is filled with 2 electrons. Then, the 2s orbital is filled with 2 electrons as well. After that, the 2p orbital is filled with 6 electrons. Moving on to the 3s and 3p orbitals, they are also filled with a total of 10 electrons.
The electron configuration continues with the 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰, and finally, the 5p⁶ orbitals.
The full ground-state electron configuration for krypton is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶.
The element symbol Cs, can be explained by the filling of electrons in the various atomic orbitals according to the Aufbau principle and the Pauli exclusion principle.
The electron configuration begins with the 1s orbital, which can hold a maximum of 2 electrons. In cesium, it is filled with 2 electrons.
Next, we move to the 2s orbital, which is also filled with 2 electrons. Then, the 2p orbital is filled with 6 electrons, distributed among its three sub-orbitals (2px, 2py, 2pz).
Moving on to the third energy level, the 3s orbital is filled with 2 electrons, followed by the 3p orbital, which is filled with 6 electrons. Continuing to the fourth energy level, the 4s orbital is filled with 2 electrons, and then the 3d orbital is filled with 10 electrons.
In the fifth energy level, the 4p orbital is filled with 6 electrons. Next, the 5s orbital is filled with 2 electrons, and then the 4d orbital is filled with 10 electrons. Finally, in the sixth energy level, the 5p orbital is filled with 6 electrons, and the last electron goes into the 6s orbital.
Therefore, the full ground-state electron configuration for cesium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s¹.
To know more about electron configuration here
https://brainly.com/question/29184975
#SPJ4
please respond and help if you’re able to

Answers
Iron (III) chloride reacts with potassium hydroxide to produce iron (III) hydroxide and
potassium chloride.
Answers
Answer:
The balanced chemical equation is :
FeCl3 + 3KOH → Fe(OH)3 + 3KCl
Câu 12. Hiện tượng nào sau đây không phải là sự nóng chảy?
A.Mỡ lợn tan ra khi đun nóng.
B.Đun nóng nến chuyển sang thể lỏng.
C.Kem bị tan chảy khi đưa ra ngoài tủ lạnh.
D. Cồn cháy sinh ra khí carbon dioxide và nước
Answers
What happens to energy when water changes states of matter from solid to gas?
Answers
Answer:
Sublimation is the process from a solid directly into a gas. The particles of a solid absorb enough energy to completely overcome the force of attraction between them. Solid carbon dioxide changes directly to the gaseous state. Therefore the state from soiid and gas can be observed.
Explanation:
Hope this helps! <3
Rank The Following Compounds In Decreasing (Strongest To Weakest) Order Of Basicity. NH2 NH2 NH2 NH2 ON I II III IV i>iii>ii>iv iii>ii>i>iv iv>iii>ii>i ii>iii>i>iv iv>ii>iii>iv
Answers
Based on the information provided, the ranking of the compounds in decreasing order of basicity is as follows:
i) NH2 NH2 NH2 NH2 (strongest base) ii) ON iii) I iv) II v) III vi) IV (weakest base) the correct ranking is iv > iii > ii > i.
To rank the compounds in decreasing order of basicity, we need to consider their ability to donate an electron pair (act as a base) in a chemical reaction. The stronger the base, the higher its basicity. Let's analyze the given options:
i) NH2 NH2 NH2 NH2
ii) ON
iii) I
iv) II
v) III
vi) IV
In general, compounds with lone pairs of electrons available for donation tend to be stronger bases. Let's examine each option:
i) NH2 NH2 NH2 NH2:
This compound consists of four amino groups (-NH2). Each amino group contains a lone pair of electrons, making it a strong base. Therefore, it is expected to be the strongest base among the given options.
ii) ON:
This compound contains an oxygen and a nitrogen atom. While both atoms have lone pairs of electrons, the oxygen atom is more electronegative, which can decrease its basicity compared to nitrogen-containing compounds.
iii) I:
This option only states the element iodine (I). Iodine is not a basic compound on its own since it does not possess a readily available lone pair of electrons for donation.
iv) II:
This option only states the Roman numeral "II" without specifying a particular compound or element, making it difficult to determine its basicity.
v) III:
This option only states the Roman numeral "III" without specifying a particular compound or element, making it difficult to determine its basicity.
vi) IV:
This option only states the Roman numeral "IV" without specifying a particular compound or element, making it difficult to determine its basicity.
To know more about basicity visit:
brainly.com/question/30518341
#SPJ11
How many moles are in 170.3 grams of Carbon?
Please help show me how you do this❤️
Answers
Answer:
There are 14.2 moles of carbon in 170.3 grams of carbon
Explanation:
The mass of one mole of carbon is known as the molar mass of carbon. the unit of molar mass is grams/mole, g/mol.
The molar mass of carbon is 12 grams. This means that for every 12 grams of carbon, one has one mole of carbon.
To determine the number of moles of carbon present in 170.3 grams of carbon, the given mass of carbon, that is 170.3 grams is divided by 12.
The formula to use is: number of moles = mass of substance/molar mass of substance
Number of moles of carbon = 170.3 g / 12 g/mol = 14.2 moles
Therefore, there are 14.2 moles of carbon in 170.3 grams of carbon
which of the following statements is not true of solutions? their solutes will settle out after long periods. they are mixtures. they are uniform. they can vary in concentration.
Answers
The statement that is not true of solutions is: "Their solutes will settle out after a long periods." Option A is correct.
This statement is false because a solution is a homogeneous mixture of a solute and a solvent, where the solute particles are evenly distributed throughout the solvent particles. A solution is a stable mixture and its solutes will not settle out after long periods of time, as long as the solution remains undisturbed.
The other statements are true of solutions: "They are mixtures" - a solution is a type of mixture that contains two or more substances that are physically combined.
"They are uniform" - a solution has a uniform composition throughout the mixture, which means that the concentration of the solute is the same in all parts of the solution.
"They can vary in concentration" - the concentration of a solution can be varied by changing the amount of solute or solvent in the solution.
Hence, A. their solutes will settle out after a long periods is the correct option.
To know more about homogeneous mixture here
https://brainly.com/question/24898889
#SPJ4
--The given question is incomplete, the complete question is
"Which of the following statements is not true of solutions? A) their solutes will settle out after long periods. B) they are mixtures. C) they are uniform. D) they can vary in concentration."--
you couldn't hear a dump truck driving through a nitroglycerin plant
Answers
It is very dangerous to hear anything loud or cause any impact or shock while working with nitroglycerin because it can cause an explosion. Therefore, you cannot hear a dump truck driving through a nitroglycerin plant due to the danger it poses to the employees and the facility.
Nitroglycerin is an explosive material that is highly sensitive to impact, heat, or friction. It is also a primary explosive, which means that it can detonate by itself without any external stimulus. This property makes nitroglycerin one of the most dangerous substances on the planet. As a result, it is always stored in a safe location and transported with the utmost care and security to prevent accidental explosions.
When working with nitroglycerin, it is essential to follow strict safety protocols and procedures to ensure the safety of the employees and the facility. Any loud noise or impact can cause a shock wave that can trigger an explosion. Therefore, it is vital to maintain a quiet environment to avoid any unnecessary risks or accidents.
You cannot hear a dump truck driving through a nitroglycerin plant because it is incredibly dangerous to do so. The vibration and noise generated by the truck can cause a shock wave that can trigger an explosion. Therefore, the facility's employees must maintain a quiet environment and avoid any loud noises or vibrations that can cause an accident or explosion.
Learn more about nitroglycerin here:
https://brainly.com/question/6347387
#SPJ11
What is the density of a block of marble that occupies 270 cm3 and has a mass of
908 g?Answer in units of g/cm3
.
Answers
The density of the block of marble will be 3.36g/cm3.
What is density?The density of a material is the measure of how tightly it is packed together. It is expressed as mass per unit volume.
Density is typically classified into two types: absolute density and relative density.
Relative density, also referred to as specific gravity, is the ratio of a material's density to the density of a reference material.
In the given scenario, the mass of the marble is 908g, and volume of the marble is 270cm3.
We know that,
Density = mass/volume.
Density = 908/270.
Density = 3.36g/cm3.
Thus, the answer is 3.36g/cm3.
For more details regarding density, visit:
https://brainly.com/question/15164682
#SPJ1
How have your ideas about evolution and its effects on people changed
Answers
As an AI language model, I don't have personal experiences or beliefs, but I can provide information on how scientific understanding of evolution and its effects on people has evolved over time.
The theory of evolution, proposed by Charles Darwin in the mid-19th century, has undergone significant advancements and refinements since its inception. Our understanding of evolution has been shaped by ongoing research, discoveries, and advancements in fields such as genetics, paleontology, and anthropology. Here are some key developments that have influenced our understanding of evolution and its effects on people:
Modern Synthesis: In the early 20th century, the Modern Synthesis, also known as the Neo-Darwinian Synthesis, integrated Darwinian natural selection with Mendelian genetics. This framework established the basis for understanding how genetic variation, mutation, and natural selection contribute to evolutionary processes.
Genetic Basis of Inheritance: The discovery of the structure of DNA and the field of molecular genetics provided insights into the mechanisms of inheritance. It revealed how genetic information is passed from generation to generation, allowing for a more detailed understanding of evolutionary processes at the molecular level.
Learn more about scientific here:
https://brainly.com/question/17152046
#SPJ11
does anyone know the substance of an egg after it has been cooked?
Answers
Answer:
Denaturation is what happens when heat is applied to the eggs. ... The heat coming from your stove denatures the protein by disrupting some of its bonds that held the molecule into shape. In the case of hard-boiled eggs, the proteins clump together and solidify, causing the egg white and yolk to harden.
I HOPE THIS WILL HELP YOUHAVE A GREAT DAY :)
Earth's surrounding atmosphere (all of the air in the world) is part of its __________.
A. biosphere
B. food chains
C. Niche
D. population
Answers
Answer:
im an idiot but i think its A
if im wrong im so sorry
Explanation:
The
is often referred to as the powerhouse of the cell since 1 point
it is where sugar is used to generate energy.
mitocondria
lysosome
ribosome
O golgi bodies
Answers
If a sample of coffee had 31.13 grams of caffeine (C8H10N4O2), how many atoms of nitrogen are present?
Answers
Find gfm of caffeine
Get moles of caffeine then moles of N
Then use avogadros number to get atoms
3.85 x10^23 atoms

When carbon bonds with oxygen, what is formed? when carbon bonds with oxygen, is formed.
Answers
The correct answer is Carbon Dioxide.
What are carbon bonds?Because each carbon is identical, they all contain four valence electrons, so they can easily bond with different carbon atoms to form extended chains or rings. A carbon atom can bond with another carbon atom two or three times to create double and triple covalent adhesives between two carbon atoms.The partial payments on the fluorine and carbon are beautiful, contributing to the unusual bond power of the carbon-fluorine bond. The bond is marked as "the strongest in organic chemistry," because fluorine forms the strongest single bond to carbon.In chemistry, a covalent bond is the strongest bond. In such bonding, every two atoms transfer electrons that bind them concurrently. For example, water molecules are bonded jointly where both hydrogen atoms and oxygen atoms transfer electrons to form a covalent bond.
To learn more about carbon bonds, refer to:
https://brainly.com/question/14700099
#SPJ4
In an experiment to study the formation of HI (g), H2 (g+ I2(g)→ 2HI (g), H2 (g) and I2(g) were placed in a sealed container and allowed to react. on one set of axes, sketch concentration vs time curves for H2 and Hi. Explain the concept of a dynamic equilbrium.
Answers
In an experiment to study the formation of HI (g), H2 (g) + I2 (g) → 2HI (g), H2 (g) and I2 (g) were placed in a sealed container and allowed to react. On one set of axes, the concentration vs time curves for H2 and HI would look like a parabola, where the highest concentration of the reactants are at the beginning, and gradually decline as the reaction reaches equilibrium. The concept of dynamic equilibrium is that the rates of the forward and reverse reaction are equal, and the concentrations of the products and reactants remain constant.
Dynamic equilibrium is a state of a chemical system in which the rate of the forward reaction is equal to the rate of the reverse reaction. In this state, the concentrations of reactants and products remain constant over time as they continue to react with each other. The system does not appear to be changing since the forward and backward reactions occur at the same rate.
The formation of HI(g) from H2(g) and I2(g) represents a reversible reaction. Initially, the concentration of H2(g) is high and the concentration of HI(g) is zero. As the reaction proceeds, the concentration of H2(g) decreases while the concentration of HI(g) increases. Once the system reaches dynamic equilibrium, the concentration of both H2(g) and HI(g) remains constant.
Learn more about dynamic equilibrium at https://brainly.com/question/12920261
#SPJ11
Help please for this problem.

Answers
1.59 mol co2 / 4 mol co2 = mol c2h2 / 2 mol c2H2
So mol of c2h2 is .79 mol
There is 27.04 grams in a mol of c2h2,
So we have 0.79*27.04=21.5 grams of c2h2
If the actual yield of sodium chloride from the reaction of
8.3 g of sodium and 4.5 g of chlorine is 6.4 g, what is the percent yield?
Answers
Answer:
86%
Explanation:
Calculate the theoretical yield from the mass of each reactant. The lesser amount is the theoretical yield. Then use the following formula to calculate the percent yield: % yield = actual yield/theoretical yield X 100.
solid iron forms when liquid iron because cooler. is this energy change a sign of a chemical change?
Answers
Solid iron forms when liquid iron because cooler. this energy change is not sign of chemical change. this is a physical change.
The physical change is the change no new substance is formed only the physical properties changes such as size, color, shape , phase change of the substance. A chemical change is a change in which a new substances id form or the chemical properties changes. in the case of iron melting the state of iron changes from solid to liquid no new substances is formed so. this type of change is physical change.
Thus, Solid iron forms when liquid iron because cooler. this energy change is not sign of chemical change. this is a physical change.
To learn more about the physical change here
https://brainly.com/question/17931044
#SPJ4
Please help me with this science quesiton

Answers
The atoms inside the reactants reorganise their chemical bonds during a chemical reaction to create products. There will therefore always be a shift in energy when chemical reactions take place.
What atomic configuration occurs throughout a chemical change?The atoms inside the reactants rearrange and link differently throughout a chemical reaction to create one or more innovative brands with properties distinct from the reactants. A chemical change occurs when a new material is created.
What chemical process causes an energy change?Exothermic refers to chemical reactions which release energy. When bonds are created in the products of exothermic processes, more energy is produced than is required to rupture the connections between the reactants. Endothermic refers to chemical reactions that either use or absorb energy.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
why does 1.00 gram of powered magnesium react faster than 1.00 gram of solid magnesium?
Answers
Ans
Higher surface area
Explanation:
The rate of chemical reacts are determined by temperature, agitation, and surface area. The more surface a substance is exposed to, the more it reacts.
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
I NEED HELP WITH THIS WHOLE PAGE

Answers
1) The endocrine glands pictured above are:
Thyroid Gland and Parathyroid glandAdrenal GrandOvariesPancreatic GrandPitutuary GlandPineal Gland.2) Organs of the endocrine system are often called glands. An organ is responsible for the production of one or more substances such as hormones, digestive juices, perspiration, tears, saliva, or milk.
The compounds are released directly into the circulation by the endocrine glands. The compounds are released by exocrine glands into a duct or aperture to the interior or outside of the body.
3) Pituitary Gland produces the following hormones:
Adrenocorticotropic hormone Follicle-stimulating hormoneGrowth hormone (GH)Luteinizing hormone (LH) etc.What is the importance of the Endocrine system?The endocrine system regulates juvenile growth and development, adult body processes, and the reproductive process. All of the body's key activities and processes are controlled and regulated by the endocrine system: Energy management. Reproduction.
The pituitary gland, for example, is located at the base of the brain and is no larger than a pea. Despite its tiny size, the pituitary gland is sometimes referred to as the "master gland." Many other endocrine glands are controlled by the hormones it produces.
Learn more about glands:
https://brainly.com/question/4133041?
#SPJ1