Answers
Answer:
Explanation:
Chemistry is the science that studies the composition, structure and properties of matter and the changes it undergoes during chemical reactions and their relationship with energy
Explanation:
Chemistry is the branch of science concerned with the substances of which matter is composed, the investigation of their properties and reactions, and the use of such reactions to form new substances.
Related Questions
How many yards are in a 100 meter race ? How many feet?
Answers
Taking into account the change of units, 100 meters are 109.361 yards and 328.084 feets.
Rule of threeThe rule of three is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them.
That is, what is intended with it is to find the fourth term of a proportion knowing the other three.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied.
To solve a direct rule of three, the following formula must be followed, being a, b and c known data and x the variable to be calculated:
a ⇒ b
c ⇒ x
So: \(x=\frac{cxb}{a}\)
The direct rule of three is the rule applied in this case where there is a change of units.
Distance in yards and feetTo perform in this case the conversion of units, you must first know that:
1 m= 1.09361 yards1 m= 3.28084 feetsThen, you can apply the following rules of three:
if 1.09361 yards is 1 meter, how many yards equals 100 meters?1 meter ⇒ 1.09361 yards
100 meters ⇒ x
So: \(x=\frac{100 metersx1.09361 yards}{1 meter}\)
Solving:
x= 109.361 yards
if 3.28084 feets is 1 meter, how many yards equals 100 meters?1 meter ⇒ 3.28084 feets
100 meters ⇒ x
So: \(x=\frac{100 metersx3.28084 feets}{1 meter}\)
Solving:
x= 328.084 feets
In summary, 100 meters are 109.361 yards and 328.084 feets.
Learn more about rule of three and change of units with this example:
brainly.com/question/12482948
#SPJ1
If a substance has a half-life of 14.9 s, and there are initially 100.0 g of the substance, how many grams will remain after precisely five minutes?
Answers
Answer:
[A] = 8.69x10⁻⁵g
Explanation:
Kinetics law of a radioactive atom, A, is:
Ln[A] = -kt + ln[A]₀
Where [A] could be taken as mass of A after time t (In seconds),
k is rate constant = ln2 / Half-Life
[A]₀ is intial mass of A
k = ln2 / 14.9s = 0.04652s⁻¹
Time in seconds:
5min * (60s / 1min) = 300s
Replacing each value:
ln[A] = -0.04652s⁻¹*300s + ln[100.0g]
ln[A] = -9.35
[A] = 8.69x10⁻⁵gHow many sigma bond and pi bond are present
Answers
5. A student measures the mass of an object three times on a balance in order to calculate the objects
density. The recorded masses are 70.21 g, 64.55 g, and 74.70 g. The accepted value for the mass of
the object is 70.21 g.
a. Are these measurements accurate? Why or why not?
b. Are the measurements precise? Why or why not?
Answers
Answer:
This is not chemistry this is physics
Which statement about balanced chemical equations is true?
OA. The mass of the new atoms that are formed equals the mass of
the atoms that made up the reactants.
OB. The total mass of the reactants equals the total mass of the
products.
OC. The total number of moles of products equals the total number of
moles of reactants,
OD. The mass of the products is greater than the mass of the
reactants when the number of moles increases.
SUBMIT
Answers
The total mass of the reactants equals the total mass of the products the statement about balanced chemical equations is true. Hence, option B is correct.
This is known as the Law of Conservation of Mass, which states that matter can neither be created nor destroyed in a chemical reaction. In other words, the mass of the reactants must equal the mass of the products in a balanced chemical equation.
While the identities of the atoms may change during a reaction, the total number of atoms of each element on both sides of the equation must be the same, thus leading to the conservation of mass.
To learn more about the balanced chemical equation, follow the link:
https://brainly.com/question/28294176
#SPJ1
Please help i need this chart filled out!!!




Answers
The volume of the solids are found in the attached table.
What are the unit for measuring volume of liquids?Volume of a substance refers to the amount of space a given mass of the substance occupies.
Since liquids have no definite volume, the volume of liquids are measured using containers.
Also, the volume of solids can be determined by displacing liquids.
The unit for measuring the volume of a liquid substance is liters.
Other multiples of the base unit are, mL, cL, dL, daL, hL, kL.
Considering the given volume of substances, the table can be filled as seen in the attachment.
In conclusion, the volume of solids was determined by displacing liquids water.
Learn more about volume of liquids at: https://brainly.com/question/26216613
#SPJ1

Which of the following structural adaptation helps an oraganism obtain food
Answers
Element
Element
-0)
18) Element Rand Element Q have the same number of valence electrons. These elements both have similar chemical behavior, but Element Rhas fewer energy levels than Element
Which statement best describes the positions of the two elements in the periodic table?
A) The two elements are in the same period, with Element R the first element in the period and
Element Q the last element.
B) The two elements are side by side in the same period, with Element Q to the left of Element R.
C) The two elements are in the same group, with Element R just above Element Q
D) The two elements are in the same group, with Element Q at the top of the group and Element Rat the bottom
O USATestprep, LLC 2021, All Rights Reserved
Read Our Blog Privacy Policy.
PHONE 1 - 877-377- 9537 I FAX 1 - 877-816-0808
ol
A UK12 D 4x
T
Answers
Answer:
the two elements are in the same period, with element R the first element in the period and element Q the last element
if an antacid tablet weighed 1.6 grams, how many moles of gastric acid (hci) would it neutralize? use the results obtained in data tables 1 and 2 to explain and quantify your answer.
Answers
The 0.015 moles of gastric acid will be neutralized by a 1.6 gram antacid pill.
By inhibiting the enzyme that produces acid in the stomach to break down food for digestion, antacids neutralize the gastric acid there. An enzyme called pepsin, which breaks down proteins, is inhibited by the antacids, which work by neutralizing the stomach's pH.
0.342 grams of HCL are neutralized every gram of antacid.
Based on the titration's equivalence point expression, this is calculated.
1.6 gram HCL neutralized antacid is,
(0.342 grams HCL to 1 grams antacid) A 1.6 gram antacid
= 0.5472 gram
HCL has a 36.5 gram molar mass.
Moles of HCL = 0.5472 g/36.51 g = 0.01499
As a result, HCL has a mole of 0.015 moles of gastric acid (hci) would it neutralize
To learn more about Antacids Please click on the given link:
https://brainly.com/question/5328009
#SPJ4
For a particular first-order reaction, it takes 48 minutes for the concentration of the reactant to decrease to 25% of its initial value. What is the value for rate constant (in s -1) for the reaction
Answers
Answer: The value for rate constant for a reaction is \(4.81\times 10^{-4} s^{-1}\)
Explanation:
The integrated rate law equation for first-order kinetics:
\(k=\frac{2.303}{t}\log \frac{a}{a-x}\) ......(1)
Let the initial concentration of reactant be 100 g
Given values:
a = initial concentration of reactant = 100 g
a - x = concentration of reactant left after time 't' = 25 % of a = 25 g
t = time period = 48 min = 2880 s (Conversion factor: 1 min = 60 s)
Putting values in equation 1:
\(k=\frac{2.303}{2880s}\log (\frac{100}{25})\\\\k=4.81\times 10^{-4} s^{-1}\)
Hence, the value for rate constant for a reaction is \(4.81\times 10^{-4} s^{-1}\)
The volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62°C. The gas is most likely __________.
A. SO2
B. SO3
C. NH3
D. NO2
E. Ne
Answers
The gas that has a volume of 752 mL at 1.98 atm and 62°C is most likely NO₂ (option D).
How to calculate volume?The volume of a sample of gas can be calculated using the following formula:
PV = nRT
Where;
P = pressureV = volume n = number of molesR = gas law constantT = temperatureAccording to this question, the volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62°C. The number of moles is as follows:
1.98 × 0.752 = n × 0.0821 × 335
1.489 = 27.5n
n = 0.054mol
molar mass of the gas = 2.49g ÷ 0.054mol = 45.99g/mol
The gaseous substance with the molar mass of 45.99g/mol is NO₂.
Learn more about moles at: https://brainly.com/question/27058396
#SPJ1
30 POINTS MATCH THE DEFINITIONS PLEASE, ITS PROBABLY EASY FOR U THO

Answers
Answer:
(going down the line)
1. matter
2. hypothesis
3. energy
4. independent variable
5. theory
6. quantitative data
7. dependent variable
8. constant
9. law
10. qualitative data
Explanation:
Question
The melting points of canola oil, corn oil, sunflower oil, and peanut oil are 10°C, -11°C, -17°C, and -2°C respectively.
You cool a mixture of these oils to 5°C.
Which oil can be separated easily?
Responses
peanut oil
sunflower oil
canola oil
corn oil
Answers
The oil that can be separated at this temperature is canola oil.
What is the melting point?The term melting point has to do with the temperature in which a solid can be converted to liquid. This is the temperature at which the various bonds that holds the molecules of the substance can be broken off.
We are told in the question that; the melting points of canola oil, corn oil, sunflower oil, and peanut oil are 10°C, -11°C, -17°C, and -2°C respectively. We want to know the oil that can be separated easily at 5°C.
Learn more about melting point:https://brainly.com/question/28902417
#SPJ1
QUESTION 54
What is/are the reactant(s) of the chemical reaction shown?
CH4 (g) + 202 (g) → CO2 (g) + 2H20 (g)
O CH4 only
O 02 only
O CH4 and 02
O CO2 and H20
The process above is not a chemical reaction
Answers
Answer:
the answer is C.
Explanation:
CH4 and 02
What is the percentage yield when 20g of aluminium are produced from 50g of aluminium oxide
Answers
Answer:
,
Explanation:
Refer to pics.............


26.4g is the percentage yield when 20g of aluminium are produced from 50g of aluminium oxide.
What is percentage yield?The % ratio of the theoretical yield to the actual yield is known as the percent yield. It is calculated as the theoretical yield increased by 100% divided by the experimental yield. The percentage return is 100% if the theoretical and actual yields are equal. Because the real yield is frequently lower than the theoretical value, percent yield is typically lower than 100%.
This may be due to incomplete or conflicting reactions or sample loss during recovery. If the percent yield is more than 100%, more sample than expected was retrieved from the reaction. This may have happened when other reactions took place and the product was also created.
2Al\(_2\)O\(_3\) → 4 Al + 3O\(_2\)
2 mole of Al\(_2\)O\(_3\) =4 mol of Al
2(2×27+48) =4×27
204g of Al\(_2\)O\(_3\) = 108g of Al
50g of Al\(_2\)O\(_3\) =x
26.4g =x
Therefore, 26.4g is the percentage yield when 20g of aluminium are produced from 50g of aluminium oxide.
To know more about percentage yield, here:
https://brainly.com/question/30774234
#SPJ2
How many grams of Na2S will be produced if you react 35.0g sodium
Answers
What are the three forest ecosystems and how are they similar? How are they different?
Answers
Answer: There are three main types of forests: tropical rainforests, deciduous forests, and coniferous forests. Tropical rainforests are found near the equator (the center of Earth), where they are warm all year round.
Explanation:
Ecosystems are defined as a geographical region where biotic factors like animals, plants and microorganisms live together and interact with the abiotic factors.
The three forest ecosystems are temperate, boreal and tropical.
What are the similarities and the differences of the forest ecosystem?Tropical forest ecosystems have high precipitation and temperature also a high humidity rate. The plant has a twelve-month growing period and the forests are found near the equator regions.
Temperate is found in between the boreal and the tropical forests, it has a high level of rainfall and humid conditions and is covered with the deciduous type of trees.
Boreal or the taiga forest ecosystems are located in the subarctic regions with low temperatures and have long winters. They are covered majorly by the scale-leaved evergreen and the needle leaves cones.
All three forest ecosystems have great species diversity, the tropical and the temperate forest have dense vegetation and the temperate and boreal have evergreen forests.
Therefore, three forest ecosystems are temperate, boreal and tropical.
Learn more about forest ecosystem here:
https://brainly.com/question/20314539
A 1 liter solution contains 0.383 M hydrofluoric acid and 0.510 M potassium fluoride.
Addition of 0.096 moles of calcium hydroxide will:
(Assume that the volume does not change upon the addition of calcium hydroxide.)
Raise the pH slightly
Lower the pH slightly
Raise the pH by several units
Lower the pH by several units
Not change the pH
Exceed the buffer capacity
Answers
Answer:
Lower the pH slightly
Explanation:
The mixture of HF, hydrofluoric acid and KF, potassium fluoride produce a buffer that is defined for the equilibrium:
HF(aq) → H⁺(aq) + F⁻(aq)
The buffer can maintain the pH of a solution despite the addition of strong bases or acids.
The reaction of HF with Ca(OH)2 is:
2HF + Ca(OH)2 → 2H2O + CaF2
That means the calcium hydroxide is decreasing the concentration of HF. Based on the equilibrium, the H+ and F- ions will decrease in order to produce more HF. As H+ is decreasing due the equilibrium and not for the addition of a strong base, the pH is decreasing slightly.
HELP ASAP PLS Is an oxygen ion and fluorine ion bigger and why?
Answers
Answer:
From top to bottom of the periodic table ions will increase in radii. However, now left to right the radius is more of a function of the number of electrons. ... Similarly, O2- will be larger than F- as both have 10 electrons but Z=8 for oxygen and Z=9 for fluorine.
The oxygen ion is bigger than the fluorine ion , the reason is explained below
What is an Atomic Structure ?
An atom is composed of electrons , protons and neutrons .
Electrons have negative charge and are grouped in different shells around the nucleus
Protons and neutrons are present in positively charged nucleus
The nucleus has the most mass .The atomic number is the number of protons which is equal to the number of electrons.
Both oxygen atom and fluorine atoms are isoelectronic , they have 10 electrons in the shell , but oxygen has 8 protons while fluorine have 9 protons in the nucleus.
It is believed that as the atomic number increases so the attraction forces of the nuclei increases , making the nucleus smaller and the overall atom smaller
while the oxygen atom on reduction forms oxide anion , which is a dianion and a fluorine atom forms fluoride ion which has a single negative charge and the ion which has more electrons have more electron electron repulsion and the size of the ion is bigger.
Hence the oxygen ion is bigger than the fluorine ion.
To know more about Atomic Structure
https://brainly.com/question/14156701
#SPJ2
At a temperature of 11.5 °C the gas occupies a volume of 0.0141 m³. Calculate the volume the gas occupies when the temperature is raised to 95.0 °C.
Answers
Taking into account the Charles's law, the gas occupies a volume of 0.0182 m³ when the temperature is raised to 95.0 °C.
Charles's lawCharles's law shows the relationship between the volume and temperature of a gas sample at constant pressure.
This law states that the volume is directly proportional to the temperature of the gas: if the temperature increases, the volume of the gas increases, while if the temperature of the gas decreases, the volume decreases.
Mathematically, Charles' law is a law that says that when the amount of gas and pressure remain constant, the ratio between volume and temperature will always have the same value:
\(\frac{V}{T}= k\)
Considering an initial state 1 and an initial state 2:
\(\frac{V1}{T1}= \frac{V2}{T2}\)
Volume in this caseIn this case, you know:
V1= 0.0141 m³T1= 11.5 C= 284.5 K (being 0 C= 273 K)V2= ?T2= 95 C= 368 KReplacing in Charles's Law:
\(\frac{0.0141 m^{3} }{284.5 K}=\frac{V2}{368 K}\)
Solving:
\(\frac{0.0141 m^{3} }{284.5 K}x368 K=V2\)
0.0182 m³= V2
Finally, the gas occupies a volume of 0.0182 m³ when the temperature is raised to 95.0 °C.
Learn more about Charles's law:
https://brainly.com/question/4147359
#SPJ1
what is 2H2O state of matter
Answers
Answer:
2 MOLECULES OF WATER
Explanation:
SO, LIQUID
2. A shopper in a supermarket takes a box of sugar from a shelf that is 1.5 m high because he is
going to bake some muffins. He also needs to get some blueberries. The sugar has a weight of 5N.
What Potential Energy did the sugar have before it was taken from the shelf?
Answers
Explanation:
kinetic energy?? idek hope I helped in anyway possible
Answer:
7.5 joules also 7.5 J
Explanation:
a 10 gram ball is rolling at 20 m/s. what energy does the ball have
Answers
Answer:
2 joule
Explanation:
10 gram= 0,01kg
The ball has kinetic enegry
K=1/2 *m *speed²
K=1/2 *0,01 * 400
K=2 Joule
Both the sweatshirt and the balloon are negatively charged. They are most
likely to (blank)
one another.
A-give
B-attract
C-repel
D-help
Answers
Answer:
B attract
Explanation:
There will be statication
Match the solution with the correct concentration.

Answers
Answer:
1. is Molar (with capital M)
2. is molal (m)
Explanation:
By definition, 1 Molar solutions have 1 mol of solute in 1 L of solution and 1 molal solutions have 1 mol of solute in 1 Kg of solvent
If an atom contains 11 protons and 12 neutrons, its ATOMIC NUMBER is: a 1 b 11 c 12 d 23
Answers
Answer:
B)11
Explanation:
The atomic number is the number of protons and electrons in an atom!!
HOPE THIS HELPS!!!!!!
A compound has a formula mass of 228.0 and an empirical formula of C2H4O3. What is the molecular formula
Answers
Answer:
C₆H₁₈O₉
Explanation:
First we calculate the molar mass of the compound represented by the empirical formula:
Molar Mass = (Molar mass of C) * 2 + (Molar Mass of H) * 4 + (Molar Mass of O) * 3Molar Mass = 12 * 2 + 1 * 4 + 16 * 3 = 76 g/molThen we divide the given formula mass by the calculated molar mass:
228 / 76 = 3Thus we multiply by 3 the subscripts in the empirical formula:
The molecular formula is C₆H₁₈O₉As the temperature in a sample of gas molecules decreases, which
of the other properties of the gas molecules could be caused to
increase or decrease? *
(Please help me)
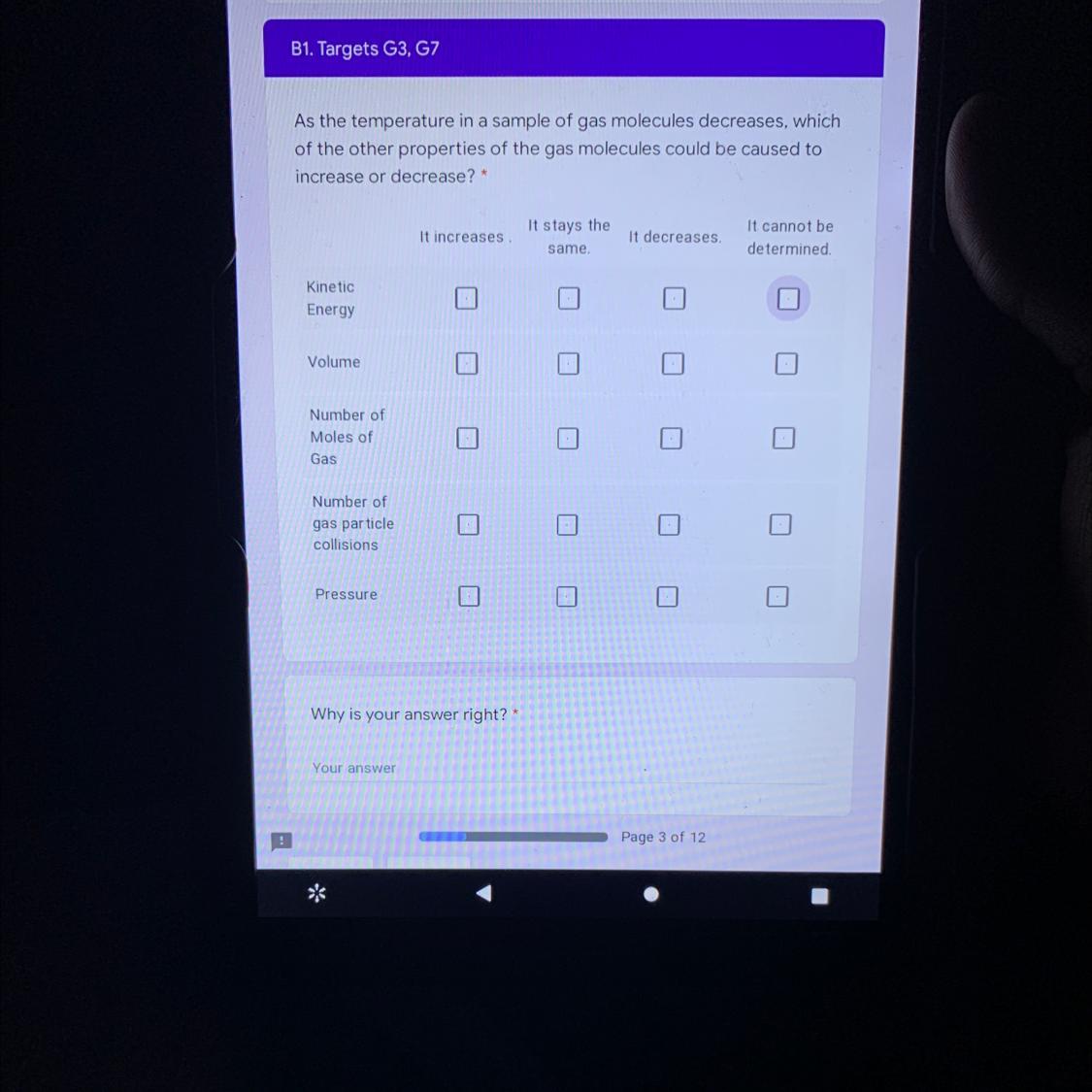
Answers
Answer:
See Explanation
Explanation:
The kinetic energy of gas molecules is related to the temperature of the gas because temperature is a measure of the average kinetic energy of the molecules of a gas hence the lower the temperature of the gas the lower the average kinetic energy of the molecules of the gas.
The volume of a given mass of gas is directly proportional its absolute temperature(Charles's law). Hence, as the temperature of the gas gas decreases, the gas volume also decreases.
The number of gas molecules is totally independent of the temperature of the gas hence it stays the same.
As the temperature decreases, the number of collision of gas particles decreases as the kinetic energy of the gas particles decreases.
The pressure of a given mass of gas is related to the movement of gas particles. Hence, the lower the temperature the lower the pressure of the gas. Pressure and temperature are directly related hence pressure decreases as temperature decreases.
Determine The Bond Angle Highlighted In Red For Each Given Molecule.

Answers
There are two characteristics of molecules, one is geometry and other is shape. Shape is excluding lone pair surrounding the central element and geometry is including the lone pair. Therefore, the angle of the given molecule can be found out by VSEPR theory.
What is VSEPR theory?
VSEPR stands for valence shell electron pair repulsions. VSEPR theory is used to predict the shape and geometry of molecules on the basis of valence electrons pairs that are present around the central element of the molecule.
According to VSEPR theory, Lone pair lone pair repulsion is greater than bond pair bond pair repulsion. There are so many limitations of VSEPR theory. There is a repulsion between bond pair electrons and lone pairs present on the central element.
a) bond angle is 180°
b)bond angle is 120°
c)bond angle is 107.28'
d)bond angle is 109.28'
Therefore, the angle of the given molecule can be found out by VSEPR theory.
To know more about VSEPR theory, here:
https://brainly.com/question/19582124
#SPJ1
What is made of two or more substances that are together in the same place but are not chemically combined
Answers
When two or more substances are together in the same place but are not chemically combined they form a mixture.
What is a mixture?The word mixture is used in chemistry to denote a combination of two or more elements that conserve their original properties, which is fundamental to achieving the separation of such elements in the future.
Therefore, with this data, we can see that a mixture is a mix or combination of two or more elements that still conserve their physical and chemical properties, thereby allowing their separation through different techniques.
Learn more about the meaning of mixture here:
https://brainly.com/question/24647756
#SPJ1