What is the first step in predicting the products of haloydrin formation?
Answers
The first step in predicting the products of halohydrin formation is to identify the alkene and the halogenating reagent.
The first step in predicting the products of halohydrin formation is to identify the alkene and the halogenating reagent. Halohydrin formation is a reaction in which an alkene reacts with a halogenating reagent, such as N-bromosuccinimide (NBS) or sodium hypochlorite (NaOCl), to form a halohydrin. The next step is to determine the mechanism of the reaction. Halohydrin formation can occur through either an electrophilic addition or a free radical addition mechanism, depending on the halogenating reagent and reaction conditions. In an electrophilic addition mechanism, the halogenating reagent acts as an electrophile, adding to the double bond of the alkene and forming a cyclic halonium intermediate. Water or another nucleophile then attacks the halonium ion, resulting in the formation of a halohydrin. In a free radical addition mechanism, the halogenating reagent generates a halogen radical, which then adds to the double bond of the alkene. A radical intermediate is formed, which then reacts with water to form the halohydrin.
In summary, the first step in predicting the products of halohydrin formation is to identify the alkene and halogenating reagent and then determine the mechanism of the reaction.
For more such questions on halohydrin
https://brainly.com/question/29662397
#SPJ11
Related Questions
What is an ionic solid?
A. A crystalline solid held together by a shared electron pool
B. A crystalline solid held together by dipole-dipole forces
C. A crystalline olid held together by covalent bonds
D. A crystalline solid held together by charge attractions
Answers
Answer:
D
Explanation:
An ionic solid is a crystalline solid held together by charge attractionsAn example is a salt crystal (Na⁺ + Cl⁻ → NaCl)An ionic solid is a crystalline solid held together by charge attractions or an ionic bond.
What is an ionic bond?
Ionic bond or electrovalent bond is a type of bond which is formed between two elements when there is an exchange of electrons which takes place between the atoms resulting in the formation of ions.
When the atom looses an electron it develops a positive charge and forms an ion called the cation while the other atom gains the electron and develops a negative charge and forms an ion called the anion.
As the two atoms are oppositely charged they attract each other which results in the formation of a bond called the ionic bond.They are good conductors of heat and electricity. They have high melting points. These compounds are brittle.
Learn more about ionic bond,here:
https://brainly.com/question/11527546
#SPJ2
which method of expressing concentration should the hydrologist use to describe the concentration of lead in the water?
Answers
Option A is the way of representing concentration that the hydrogeologist should employ to describe the density of lead in the water.
Ppm or ppb. The following details should be taken into account Ppm stands for parts per million. Ppb stands for parts per billion. • We know that one part in a million equals one part in a billion. • The same method should be employed for water concentration. 1 Water concentrations can also be expressed in fractions of a million (ppm) or parts for every billion (parts per billion) (ppb). This relationship can be used to convert between parts per million and parts per billion: 1 part in a thousand equals 1,000 parts in a billion.
Learn more about concentrations here:
https://brainly.com/question/10725862
#SPJ4
The complete question is:
Which method of expressing concentration should the hydrologist use to describe the concentration of lead in the water?
A) ppm or ppb
B) volume percent
C) mass-volume percent
D) mass percent
which is the only element in group 1 on the periodic table that forms covalent bonds?
Answers
Answer:
the answer is A.Hydrogen,I just took the test
Explanation:
What factors are included on a phase diagram?
O A. Temperature and pressure
O B. Pressure and volume
O C. Mass and volume
O D. Heat and kinetic energy
Answers
Answer:
A. Temperature and pressure
Explanation:
The factors included in a phase diagram are the temperature and pressure conditions.
A phase diagram is a combined plot of three curves all in equilibrium;
Solid- liquid, liquid -gas, and solid -gas plots
On the vertical axis, we usually have the pressureOn the horizontal axis is where the temperature is represented. Most phase diagrams are used in chemistry and mineralogy to show areas in which different phases can exist at equilibrium.The factors included on a phase diagram are temperature and pressure and the correct option is option A.
A phase diagram is a graphical representation that shows the different phases of a substance (solid, liquid, and gas) as a function of temperature and pressure. It provides valuable information about the conditions at which different phases can exist and the boundaries between them.
The temperature is typically plotted on the horizontal axis, while the pressure is plotted on the vertical axis.
By examining a phase diagram, one can determine the melting point, boiling point, and critical point of a substance, as well as the regions of stability for each phase. Additionally, phase diagrams can reveal the existence of phase transitions such as melting, boiling, and sublimation.
Thus, the ideal selection is option A.
Learn more about Phase diagram, here:
https://brainly.com/question/34149833
#SPJ6
The work of expanding a gas. Compute the total work performed when expanding an ideal gas, at constant temperature, from volume V to 2V.
Answers
The total work performed is nRT * 0.693
The work done by an ideal gas during an expansion at a constant temperature from volume V to 2V can be calculated using the equation work = nRT * ln(Vf/Vi).
Where n is the number of moles of gas, R is the ideal gas constant, T is the temperature, Vf is the final volume and Vi is the initial volume.
In this case, the initial volume is V and the final volume is 2V, so
the work done is nRT * ln(2V/V).
Since ln(2) is equal to 0.693, the work done is nRT * 0.693. Keep in mind, this equation is only true for certain types of gas at specific temperatures and pressures.
Read more about ideal gas:
https://brainly.com/question/15634266
#SPJ4
A student titrated a 50.0 mL of 0.15 M glycolic acid with 0.50 M NaOH. Answer the following questions. (21 points) a. What is the initial pH of the analyte? Ka of glycolic acid is 1.5 x 10-4 b. The student added 15.0 mL of NaOH to the analyte and measured the pH. What is the new expected pH? c. Additionally, to the previous solution, question b, 10.0 mL of NaOH was added. What is the new PH? Show your work and submit it to the last question as pdf or picture file. All answers should be 2SF. No work = No credits Calculate the molar solubility of Pb(OH)2 in the following solution. Ksp = 1.2 x 10-15 (21 points) a) in Pure water b) in 0.30 M PbCl2 c) in 0.10 M NaOH Show your work and submit it to the last question. SF = 2
Answers
a. The initial pH of the analyte is approximately 2.21.
b. The new expected pH after adding 15.0 mL of NaOH is 7 (neutral).
c. The new pH after adding 10.0 mL of NaOH is approximately 2.51 (acidic).
Determine what is the initial pH of the analyte?a. The initial pH of the analyte can be calculated using the dissociation constant (Ka) of glycolic acid. Given that the Ka of glycolic acid is 1.5 x 10⁻⁴, we can set up an equilibrium expression for the dissociation of glycolic acid as follows:
Ka = [H⁺][A⁻] / [HA]
Assuming the initial concentration of glycolic acid ([HA]) is 0.15 M and neglecting the x value (change in concentration) compared to the initial concentration, we can write:
Ka = [H⁺][A⁻] / 0.15
Since glycolic acid is a monoprotic acid, the concentration of [H⁺] will be equal to the concentration of [A⁻] (once it fully dissociates). Therefore, we can substitute [H⁺] with x in the equation above:
Ka = x² / 0.15
Solving for x, we find:
x = √(Ka * 0.15)
Substituting the given values, we have:
x = √(1.5 x 10⁻⁴ * 0.15) ≈ 0.0061
Taking the negative logarithm of x to find the pH:
pH = -log[H⁺] = -log(0.0061) ≈ 2.21
The initial pH is calculated based on the dissociation constant (Ka) of glycolic acid. Using the equilibrium expression and assuming complete dissociation, we can derive an equation relating Ka, [H⁺], and [A⁻]. Solving for [H⁺] and taking the negative logarithm gives us the initial pH value. In this case, the initial pH is approximately 2.21.
Determine what is the new expected pH?b. The addition of 15.0 mL of NaOH to the analyte will result in a neutralization reaction between the acid and the base. The moles of glycolic acid can be calculated as follows:
moles of glycolic acid = initial concentration of glycolic acid * initial volume of glycolic acid = 0.15 M * 0.050 L = 0.0075 moles
Since glycolic acid and NaOH react in a 1:1 ratio, the moles of NaOH added can be calculated as follows:
moles of NaOH = concentration of NaOH * volume of NaOH added = 0.50 M * 0.015 L = 0.0075 moles
Since the moles of glycolic acid and NaOH are equal, they will react completely, resulting in a neutral solution. Therefore, the new pH is expected to be 7 (neutral).
The addition of NaOH to the analyte leads to a neutralization reaction between the acid (glycolic acid) and the base (NaOH). The moles of each substance can be calculated using their respective concentrations and volumes. As the moles of glycolic acid and NaOH are equal in this case, they will react completely, resulting in a neutral pH of 7.
Determine how to find the new PH?c. Additionally, adding 10.0 mL of NaOH to the solution from question b will result in further neutralization of the remaining glycolic acid. Using the same approach as in question b, the moles of NaOH added can be calculated as follows:
moles of NaOH = concentration of NaOH * volume of NaOH added = 0.50 M * 0.010 L = 0.005 moles
Since the initial moles of glycolic acid were
0.0075 moles and the moles of NaOH added are now 0.005 moles, there will be an excess of glycolic acid remaining. Therefore, the solution will be acidic. To calculate the new pH, we need to determine the concentration of the remaining glycolic acid:
remaining moles of glycolic acid = initial moles - moles of NaOH added = 0.0075 - 0.005 = 0.0025 moles
The remaining concentration can be calculated as follows:
remaining concentration = remaining moles / total volume of solution = 0.0025 moles / (0.050 L + 0.015 L + 0.010 L) = 0.025 M
Using the dissociation constant (Ka) and the concentration of the remaining glycolic acid, we can set up an equilibrium expression:
Ka = [H⁺][A⁻] / [HA]
Assuming the remaining concentration of glycolic acid ([HA]) is 0.025 M and neglecting the x value compared to the initial concentration, we can write:
Ka = [H⁺][A⁻] / 0.025
Since glycolic acid is a monoprotic acid, the concentration of [H⁺] will be equal to the concentration of [A⁻]. Therefore, we can substitute [H⁺] with x:
Ka = x² / 0.025
Solving for x, we find:
x = √(Ka * 0.025)
Substituting the given values, we have:
x = √(1.5 x 10⁻⁴ * 0.025) ≈ 0.0031
Taking the negative logarithm of x to find the pH:
pH = -log[H⁺] = -log(0.0031) ≈ 2.51
Adding NaOH to the solution in question b results in further neutralization of the remaining glycolic acid. The moles of NaOH added are calculated using the concentration and volume of NaOH. Since the initial moles of glycolic acid are higher than the moles of NaOH added, there will be some glycolic acid remaining, making the solution acidic.
To determine the new pH, we need to calculate the concentration of the remaining glycolic acid and use the dissociation constant (Ka) to set up an equilibrium expression.
Solving for [H⁺] and taking the negative logarithm gives us the new pH value. In this case, the new pH is approximately 2.51.
To know more about monoprotic acid, refer here:
https://brainly.com/question/31116483#
#SPJ4
An Anti-Smoking Campaign claims the average time it takes smokers to quit smoking is 16 years. Suspecting this is incorrect, researchers take a sample of 25 former smokers and record the amount of time (in years) that it took each to quit smoking. Given a population standard deviation of 4.06, is there enough evidence to reject the campaign's claim at α=0.05?
11.2 15.8 11.2 12 8.2
10.1 14.7 10.5 13.5 12.8
12.5 14 18.8 18.6 14.9
11.8 18.2 11.3 15 16.1
19 11 9 22.1 25
Table: Years to Quit Smoking
State the null and alternative hypothesis in parts a & b. (Fill in <, >, ≤, ≥, =, or ≠ , then the value.)
a) H0: μ
b) Ha: μ
c) Is this a right-tailed, left-tailed, or two-tailed test?
d) Find the p-value.
p=
e) Should you reject or fail to reject the null hypothesis?
f) conclusion: At the 1% level of significance, there (is or is not) sufficient evidence to reject the claim.
Answers
The null hypothesis (H0) states that the average time it takes smokers to quit smoking is greater than or equal to 16 years, while the alternative hypothesis (Ha) suggests that the average time is less than 16 years.
What are the null and alternative hypotheses in the given scenario?In the given scenario, the null and alternative hypotheses are as follows:
a) H0: 16 (The average time it takes smokers to quit smoking is greater than or equal to 16 years)
b) Ha: 16 (The average time it takes smokers to quit smoking is less than 16 years)
c) This is a left-tailed test because the alternative hypothesis (Ha) suggests a decrease in the average time to quit smoking.
d) To find the p-value, we need to conduct a statistical test. The test statistic (t-value) can be calculated using the sample mean, population standard deviation, sample size, and the hypothesized population mean (16 years). Using the t-distribution and degrees of freedom (n-1 = 24), the p-value can be determined.
e) Based on the p-value obtained from the test, we compare it to the significance level (α = 0.05). If the p-value is less than α, we reject the null hypothesis. Otherwise, we fail to reject the null hypothesis.
f) In the conclusion, we state whether there is sufficient evidence to reject the claim. If the p-value is less than the significance level (α = 0.01), we can conclude that there is sufficient evidence to reject the claim. However, if the p-value is greater than α, we fail to reject the claim.
Learn more about null hypothesis
brainly.com/question/30821298
#SPJ11
Chemical analysis shows that citric acid contains 37.51% C, 4.20% H, and 58.29% O. What is the empirical formula for citric acid?
Answers
The empirical formula for the citric acid is C₆H₈O₇
Data obtained from the question Carbon (C) = 37.51%Hydrogen (H) = 4.20%Oxygen (O) = 58.29%Empirical formula =?Divide by their molar mass
C = 37.51 / 12 = 3.126
H = 4.2 / 1 = 4.2
O = 58.29 / 16 = 3.643
Divide by the smallest
C = 3.126 / 3.126 = 1
H = 4.2 / 3.126 = 1.34
O = 3.643 / 3.126 = 1.17
Multiply through by 6 to express in whole number
C = 1 × 6 = 6
H = 1.34 × 6 = 8
O = 1.17 × 6 = 7
Thus, the empirical formula for the citric acid is C₆H₈O₇
Learn more about empirical formula:
https://brainly.com/question/24818135
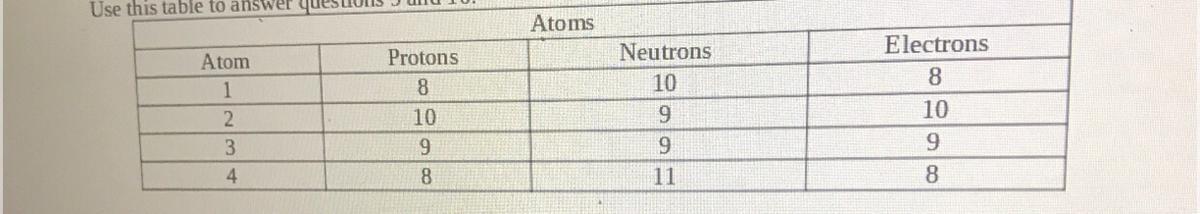
Which two are isotopes of one another?
a. Atoms 1 & 2
b. Atoms 2 & 3
c. Atoms 1 & 3
d. Atoms 1 & 4
Answers
what chemical in the catabolism of glucose enters the mitochondria?
Answers
The chemical in the catabolism of glucose that enters the mitochondria is pyruvat.
In the catabolism of glucose, the process begins with glycolysis, which takes place in the cytoplasm of the cell. During glycolysis, glucose is broken down into two molecules of pyruvate through a series of enzymatic reactions. Pyruvate is a three-carbon compound (C₃H₄O₃) and serves as an intermediate product in glucose metabolism.
After glycolysis, if oxygen is available, pyruvate enters the mitochondria, where further oxidation occurs through a process called aerobic respiration. In the mitochondria, pyruvate undergoes decarboxylation, producing acetyl CoA, which then enters the citric acid cycle (also known as the Krebs cycle). The citric acid cycle generates high-energy electrons, which are then used in the electron transport chain to produce ATP through oxidative phosphorylation.
learn more about pyruvate here:
https://brainly.com/question/31495806
#SPJ4
In the following experiments, identify the
independent and dependent variable.
The tire pressure
____ variable)
increases as the temperature increases
(____variable).
Answers
Answer: the tire pressure (dependent variable) increases as the temperature increases (independent variable)
Explanation: just did the quiz
Answer:
Person above is correct, second part is:Adding salt (independent variable) causes the boiling
temperature of the water (dependent variable) to increase.
which of the following is a correct prediction of the chemical shifts for the signals in the 1h nmr spectrum for the following compound? the structure of a molecule with the smiles string coccoc. the protons on the first carbon are labeled as roman numeral i. the protons on the second carbon are labeled as roman numeral ii. the protons of the third carbon are labeled as roman numeral iii. the protons of the fourth carbon are labeled as roman numeral iv. select answer from the options below i
Answers
The compound with the SMILES string COCCOC has the structure: CH3-O-CH2-CH2-O-CH3. In this molecule, the protons are labeled as follows:
I. Protons on the first carbon (CH3)
II. Protons on the second carbon (CH2)
III. Protons on the third carbon (CH2)
IV. Protons on the fourth carbon (CH3)
The correct order of increasing chemical shifts is I < II ≈ III < IV.
In a 1H NMR spectrum, chemical shifts depend on the electron density around the protons and their environment. Here is a correct prediction of the chemical shifts for the signals:
I. Protons on the first carbon (CH3) will have the lowest chemical shift, as they are adjacent to an electron-rich oxygen atom, which decreases the electron density around the protons.
II. Protons on the second carbon (CH2) will have a higher chemical shift than I, due to being further away from the oxygen atom, but will still be relatively low as they are adjacent to a CH3 group.
III. Protons on the third carbon (CH2) will have a similar chemical shift to II, as their environment is quite similar.
IV. Protons on the fourth carbon (CH3) will have the highest chemical shift, as they are furthest from the oxygen atoms and adjacent to a CH2 group.
Therefore, the correct order of increasing chemical shifts is: I < II ≈ III < IV.
To know something about the chemical shift, click below.
https://brainly.com/question/31321646
SPJ11
Considering the stereochemistry of the inteediate I below, which of the products would you expect. Explain your answer.
Answers
The expected product is (R)-2-bromobutane.
Stereochemistry plays a crucial role in determining the outcome of chemical reactions. In the given question, the stereochemistry of the intermediate I needs to be considered to determine the expected product.
The intermediate I indicates a chiral carbon center, denoted by an asterisk (*), which means it has four different substituents attached to it. This chiral carbon results in two possible stereoisomers: (R)-2-bromobutane and (S)-2-bromobutane.
When a reaction occurs at a chiral carbon, the stereochemistry of the reactant is usually retained in the product, assuming no racemization or inversion takes place during the reaction. In this case, the intermediate I has an (R) configuration, which implies that the product will also have an (R) configuration.
Therefore, the expected product is (R)-2-bromobutane.
Learn more about (R)-2-bromobutane.
brainly.com/question/17031230
#SPJ11
B. Organic sedimentary rock
C. Chemical sedimentary rock
6. What type of sedimentary rock is fossiliferous limestone?
A. Clastic sedimentary rock
B. Organic sedimentary rock
C. Chemical sedimentary rock
Answers
Answer:
A. Clastic sedimentary rocks
Explanation:
Chemical form = CaCO3
Clastic sedimentary rocks form from the accumulation and lithification of mechanical weathering debris. Examples include: breccia, conglomerate, sandstone, siltstone, and shale.
Hope this helps! :)
45.0 yard to kilometer
Answers
Answer:
0.0411 kilometre
Explanation:
1 yard = 0.0009144
Answer:
0.0411 kilometre
hope it helps :)
Match each scientist to their discovery regarding the atom
Thomson
Electrons have a charge of -1.
Rutherford
Atoms are indivisible
Millikan
Atoms have a positive nucleus
Dalton
Atoms contain electrons.
Answers
Answer:
Thomson--atoms cotain electron
Ernest Rutherford--atoms have a positive nucleus
R.A Millikan--electrons have Q=-1
Dalton--atoms are indivisible
Millikan---> Electrons have a charge of -1
Rutherford ---> Atoms have a positive nucleus
Thomson -----> Atoms contain electrons
Dalton --------> Atoms are indivisible
The atomic theory went through several modifications and different scientists proposed various models of the atom until our present conception of the atom was developed.
The atom was first defined as the smallest indivisible particle of a substance. This idea of "indivisibility" of the atom stems from Dalton's theory.
The fact that atoms were composed of negatively charged electrons was proven by the experiments of J.J Thompson using the cathode ray tube. Millikan's charge to mass experiment showed that the electron has a charge of -1.
Rutherford, in his famous gold foil experiment showed that atoms were composed of a positively charged nucleus.
Learn more: https://brainly.com/question/1596638

24. How many grams of NaCl are required to prepare 60ml of a 75% solution?
Answers
Considering the definition of mass volume percentage, a mass of NaCl of 45 grams is required to prepare 60ml of a 75% solution.
Definition of mass volume percentageA mass volume percentage is a ratio of the mass of a solute to the volume of the solution, expressed as a percentage. The mass/volume percentage is calculated as the mass of solute divided by the volume of solution:
mass volume percentage= (mass of solute÷ volume of solution)×100%
Mass of NaClIn this case, you know:
mass volume percentage= 75%mass of solute=?volume of solution= 60 mLReplacing in the definition of mass volume percentage:
75% grams/mL= (mass of solute÷ 60 mL)×100%
Solving:
75% grams/mL÷100%= mass of solute÷ 60 mL
0.75 grams/mL= mass of solute÷ 60 mL
0.75 grams/mL×60 mL= mass of solute
45 grams= mass of solute
Finally, a mass of NaCl of 45 grams is required.
Learn more about mass volume percentage:
brainly.com/question/13155665
#SPJ4
Calculate the volume of water which was liberated during the decomposition of hydrogen peroxide.
Answers
The answer is :
The normal equation for the decomposition of hydrogen peroxide indicates that 2 vol. peroxide vapor should give rise to 2 vol. water vapor and 1 vol. oxygen.
What is hydrogen peroxide ?
Hydrogen peroxide is water (H2O) with an extra oxygen molecule (H2O2).The extra oxygen molecule oxidizes, which is how peroxide gets its power says Dr. Beers. This oxidation kills germs and bleaches color from porous surfaces like fabrics. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases.To learn more about Hydrogen peroxide visit: https://brainly.com/question/18709693?
#SPJ4
below shows Eddie fishing.
Using the picture, which of the following is the best example of mechanical energy?
Group of answer choices
the pole, line, and hook used for fishing
Eddie reeling in a fish that he has caught
Eddie standing on the side of the river
the Sun shining and warming the water
Answers
Answer:
B is correct
Explanation:
pls Brainliest
Answer:
Eddie reeling in a fish that he has caught
Explanation:
Complete the sentences to explain your choice. Match the words in the left column to the appropriate blanks in the sentences on the right. more oxygen atoms less electronegative atoms fewer oxygen atoms more electronegative atoms When comparing HNO3 and HNO2, HNO, is a stronger acid because it has _______ When comparing HCIO, and HCIO, HCIO, is a stronger acid because it has _______ When comparing HCIO, and HBrO, HCIO, is a stronger acid because it has _______ When comparing CCI,COOH and CBr, COOH, CCI,COOH is a stronger acid because it has _______
Answers
As an atom's size shrinks, its electronegativity rises. This is due to the fact that electronegativity and atomic size are inversely related. Because of this, the atomic size decreases as electronegativity rises.
The contact between the nucleus and the surrounding electrons is reduced as the atomic radius rises, which results in a decline in electronegativity.
When comparing HNO3 and HNO2, HNO3 is a stronger acid because it has more electronegative atoms (in this case, more oxygen atoms).
When comparing HCIO and HCIO2, HCIO2 is a stronger acid because it has more electronegative atoms (in this case, more oxygen atoms).
When comparing HCIO and HBrO, HCIO is a stronger acid because it has fewer electronegative atoms (in this case, fewer oxygen atoms).
When comparing CCI3COOH and CBr2COOH, CCI3COOH is a stronger acid because it has more electronegative atoms (in this case, more chlorine atoms).
Learn more about electronegativity here
https://brainly.com/question/13557134
#SPJ11
look at the three solid blocks made of the same material and with the exact same dimensions which block has the greatest mass
Answers
Answer:
where are the solid blocks?
Explanation:
Consider the balanced reaction below:

Answers
Hint: Tetrafluoromethane, otherwise called carbon tetrafluoride or R−14
, is the most straightforward perfluorocarbon (CF4
). As its IUPAC name shows, tetrafluoromethane is the per fluorinated partner to the hydrocarbon methane. It can likewise be named a haloalkane or halomethane.
Complete step by step answer:
On the off chance that you take a gander at the condition, you will see that for each one mole of C
that is responded, one mole of CF4
is delivered. Thus, 1.46
moles of CF4
are created (expecting that there is sufficient F2
). A mole of CF4
has a mass of around 88
grams, so 1.46
moles is equivalent to 128.48
grams. Adjusted to critical figures, your answer will be 128
grams.
Let’s do it bit by bit:
First Change moles of carbon to moles of CF4
n(CF4)=1.46 mol C × (1 mol CF41 mol C) = 1.46 mol CF4
At that point convert moles of CF4
to grams of CF4
utilizing the molar mass
M(CF4)=(1×12+4×19)gmol−1=88gmol−1
m(CF4)=nM=1.46mol×88gmol−1=128g
So, the required answer is [128g]
When ions are tightly surrounded by solvent molecules they are said to be _____.
Answers
When ions are tightly attached to solvent molecules they are called as solvated ions.
When separation occurs, the solute is divided into ions or molecules, and every ion or molecule is surrounded by a solvent. The bonding between the solute particles and solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent molecules. For example, when we add NaCl to water, the NaCl molecules split into Na⁺ and Cl⁻ ions. These ions of sodium and chlorine then get surrounded by water molecules. We can call the ion water mixture a solution and the surrounded ions of sodium and chlorine as solvated ions.
To learn more about solvent molecules:
https://brainly.com/question/13836544
#SPJ4
To two decimal places, what is the mass in grams of one atom of helium? express your answer in proper scientific notation.
Answers
Answer:
6.64 x 10^-24 gm
Explanation:
One mole ( 6.022 x 10^23 atoms) of Helium = 4.00 gm
4.00 gm / 6.022 x 10^23 = .664 x 10^-23 gm = 6.64 x 10^-24 gm
Which of the following has the largest radius?
A. N a^ +
B. K ^ +
C. Na
D. K
Answers
K (Potassium) has the largest radius
Atomic radius – Distance between the nucleus of an atom to its outermost orbit.
In the periodic table, as we go down the group the atomic radius increases. This is because the energy level increases as we go down the group.
Na has 11 electrons. It would have 3 shells – 2,8,1
K has 19 electrons. It would have 4 shells – 2,8,8,1
Na⁺ and K⁺ are ions with the loss of electrons of the atom Na and K respectively.
Na⁺ and K⁺ would have lost one electron resulting in 2 and 3 shells respectively. Thus, they are smaller than Na and K respectively.
Na and K belongs to period 3 and 4 down the group. So, K in 4th period would have more energy levels compares to Na. Thus, K (Potassium) would have the largest atomic radius with 4 shells.
To know more about Atomic radius
https://brainly.com/question/238050
#SPJ1
Convert 48.66 L to daL
Answers
As a result of this process, the proportions of oxygen and carbon dioxide in
air breathed in and air breathed out change.
Which one of the statements is true? Tick the correct box. [1]
- Air breathed out has less carbon dioxide and more oxygen than air breathed in.
- Air breathed out has less carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and more oxygen than air breathed in.
Answers
Answer:
the third one
Explanation:
When you breathe in, you inhale oxygen and exhale carbon dioxide
How can we differentiate between table salt solution lemon juice and soap solution
Answers
Table salt solution is neutral in nature. Lemon juice solution is more acidic in nature and soap solution is slightly basic in nature.
What is the solution ?Any combination of one or more solutes that have been dissolved in a solvent is referred to as a solution. To create a homogenous mixture, a solute must dissolve in a solvent. To create a homogenous mixture, a solute must dissolve in a solvent.
The solution of soap is alkaline. Alkaline has a pH greater than 7. While lemon juice has an acidic pH of roughly 2, during metabolism it actually becomes alkaline and has a pH much above 7.
Sodium chloride, or table salt, is the original "salt." A neutral salt is sodium chloride, which is produced by neutralizing sodium hydroxide and hydrochloric acid.
Thus, In this way we can differentiate between table salt solution lemon juice and soap solution.
To learn more about the solution,follow the link;
https://brainly.com/question/30665317
#SPJ9
How many km are in 1.29 x 10 ^-2 cm?
can someone pls help me
Answers
Answer: 0.0129
Explanation:
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4