What is the hydronium ion (H3O+) concentration of an aqueous HCl solution that has a pOH of 9.040?
a. 7.01 x 10-3
b. 1.10 x 10-5
c. 4.96 x 10-8
d. 3.98 x 10-10
e. 9.12 x 10-10
Answers
The hydronium ion (H3O+) concentration of the aqueous HCl solution is 4.96 x 10^-5, the correct option is b.
To determine the hydronium ion (H3O+) concentration of an aqueous HCl solution with a pOH of 9.040, we need to use the relationship between pOH and pH, which is:
pOH + pH = 14
Thus, if the pOH is 9.040, then the pH is:
pH = 14 - 9.040
pH = 4.96
Next, we can use the pH value to determine the H3O+ concentration using the following equation:
pH = -log[H3O+]
Rearranging the equation gives:
[H3O+] = 10^-pH
Substituting the pH value of 4.96 gives:
[H3O+] = 10^-4.96
[H3O+] = 4.96 x 10^-5
Therefore, the hydronium ion (H3O+) concentration of the aqueous HCl solution is 4.96 x 10^-5, which corresponds to option b.
To know more about hydronium ion click here:
https://brainly.com/question/13387755
#SPJ11
Related Questions
1 point
2. If a 10.0 L balloon expands to 20.0 L when the pressure is at 2.0 atm,
then determine the final pressure of the balloon as temperature is held
constant
Answers
Answer:
1 atmExplanation:
The final pressure can be found by using the formula for Boyle's law which is
\(P_1V_1 = P_2V_2\)
Since we are finding the new pressure
\(P_2 = \frac{P_1V_1}{V_2} \\\)
From the question we have
\(P_2 = \frac{10 \times 2}{20} = \frac{20}{20} \\ \)
We have the final answer as
1 atmHope this helps you
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
63) How many moles of CaBr
2
are in 5.0 grams of CaBr
2
2.
4.0 x 101 mol
2.5 x 10-2 mol
4.2 x 10-2 mol
1.0 x 10'mol
Answers
Answer:
I think it will be the answer
2.5 x 10-2 mol
I hope it help you
Moles to grams
1_Mg + _2_HCI ---> _1_MgCl2 + _1_H2
What mass of HCl is consumed by the reaction of 1.25 moles of magnesium?
Answers
Answer:
2.5 moles HCl = 2.5moles x 36g/mole HCl = 90 grams HCl.
Explanation:
From equation ratios the reaction ratio is 1 mole Mg to 2 moles HCl. That is, given 1.25 moles Mg, moles HCl consumed will be 2(1.25)moles, or 2.5 moles HCl = 2.5moles x 36g/mole HCl = 90 grams HCl.
9. The verb corrode means 'wear away gradually, usually by a chemical reaction." A metal
that is prized for its "resistance to corrosion" has what property?
A metal that is prized for its resistance to corrosion has what property?
Answers
Answer:
The metal has a high resistance to corrosion, meaning it is more durable and will nor rust easily.
Explanation:
The verb "corrode" means the condition of being damaged easily. In other words, it means that the metal or item gets worn out easily and is not long-lasting.
So, when metal is prized for its resistance against corrosion, it means that the metal is strong and does not wear out easily. It can outstand and withheld the corrosion effect longer. This means that the metal is durable and will not corrode easily.
2. What type of compound is water (H20) and why is it called a universal solvent?
Answers
Answer:
H2O is a covalent compound and is called a universal solvent because it dissolves most substances than any other known liquid
The universal solvent, H₂O, is a covalent molecule that dissolves more compounds than any other liquid.
Briefing:Water makes an excellent solvent because of its physical and chemical composition. In water molecules, hydrogen and oxygen atoms are organized polarly, with hydrogen possessing a positive electric charges and oxide having a negative electrical charge.
Why water is called universal solvent ?Water is referred to be the "universal solvent" because it has a wider range of dissolving abilities than any other liquid. Every living thing on the world requires this. It indicates that wherever water flows, whether through the air, the ground, or our bodies, it carries valuable molecules, minerals, and nutrients.
To know more about Universal solvent visit:
https://brainly.com/question/2166468
#SPJ2
If Earth is in between the sun and the Moon in both Image 1 and Image 2, why do you think a lunar eclipse is only happening in Image 2?

Answers
Answer:
In image 1, the moon is in a certain spot in its tilted elliptical orbit, so it is effectively under the Earth from the Sun's perspective. But in image 2, The moon is actually behind the Earth from the Sun's perspective.
Explanation:
I kinda' explained in my answer
In image 2, the lunar eclipse because the earth's shadow falls on the surface of the moon when Earth, moon, and sun all are aligned in a straight line.
What is a lunar eclipse?A lunar eclipse usually takes place when the Moon moves into the shadow of the earth. This eclipse happens only when the Sun, Earth, and Moon are exactly aligned with Earth between the other two, which can occur only on the night of a full moon when the Moon is close to the lunar node.
When the moon is eclipsed by the Earth, it gets on a reddish color that is caused by the planet when it fully blocks direct sunlight from reaching the Moon's surface, as only the light reflected from the lunar surface has been refracted by the atmosphere of the earth. Due to the Rayleigh scattering of blue light, this light appears reddish.
A total lunar eclipse lasts up to nearly 2 hours, lunar eclipses are safe to view. Therefore, image 2 represents the lunar eclipse.
Learn more about lunar eclipses, here:
https://brainly.com/question/
#SPJ2
Rank the following species in order of decreasing boiling point (highest to lowest): O3, N2, H2,CO2, O2
Answers
The following species can be listed in order of decreasing boiling point:
CO₂>O₃>N₂>O₂>H₂
The boiling point of gases depends upon the strength of the intermolecular forces of attraction acting between them and the molecular weight of the gaseous species.
CO₂ has polar bonds and also exhibits dipole - dipole interactions.
O₃ also has polar covalent bonds
O₂, N₂ and H₂ are non polar but have london dispersion forces as weak intermolecular forces.
Thus, the order of decreasing boiling point will be -
CO₂>O₃>N₂>O₂>H₂
Learn more about Boiling point, here:
https://brainly.com/question/2153588
#SPJ1
There are more than ____ STDs.
Answers
Answer:
More than 30
Explanation:
There are more than More than 30 STDs
In the first 85.0 s of this reaction, the concentration of no dropped from 1.12 m to 0.520 m . calculate the average rate of the reaction in this time interval.
Answers
In the first 85.0 s of this reaction, the concentration of no dropped from 1.12 m to 0.520 m .
What is rate of a reaction?
The speed at which a chemical reaction takes place is the rate of the reaction. It is the concentration change per unit time of a reactant in a reaction.
Since the concentration of NO reduces to half its initial concentration in 85 seconds that is from 1.12m to 0.520m, it can be said that 85 seconds is the half life interval for the reaction, Hence on average, half reaction is completed in the time interval of 85 seconds.
To learn more about rate of a reaction from the given link below,
https://brainly.com/question/12172706
#SPJ4
A snicker bar has 300. Kcal, how many Joules of energy does it contain?
Answers
Answer:
1255200J
Explanation:
1 cal = 4.184 J
1kcal = 4184 J
thus, 300 kcal= 4184 × 300
= 1255200J
= 1255.2 kJ
= 1.2552 MJ
The titration of 25. 0 ml of an unknown concentration h 2so 4 solution requires 83. 6 ml of 0. 12 m lioh solution. What is the concentration of the h 2so 4 solution (in m)?.
Answers
The calculated concentration of the H₂SO₄ solution is 0.20 M.
How to calculate the unknown concentration via titration?Firstly, we can calculate number of moles of LiOH that are present in the 83.6 mL of 0.12 M LiOH solution.
Volume in mL = 83.6 mL
Volume in litres = 83.6 / 1000 = 0.0836 L
Molarity of LiOH = 0.12 M
Now, to calculate the moles of LiOH 83.6 mL of 0.12 M LiOH solution, formula is:
Mole = Molarity × Volume
So, Moles of LiOH = 0.12 × 0.0836 = 0.010032 moles
Next, we have to calculate the amount of H2SO4 required to react with 0.010032 moles of LiOH:
2LiOH + H₂SO₄ → Li₂SO₄ + 2H₂O
From the above balanced equation, it can be said that 2 moles of LiOH reacted with 1 mole of H₂SO₄. Thus,
The number of moles of H₂SO₄ that are required to react with 0.010032 moles of LiOH = 0.010032 / 2 = 0.005016 mole of H₂SO₄
Now, finally, we will calculate the molarity of H₂SO₄:
Molarity = Mole / volume
Mole of H₂SO₄ = 0.005016 mole
Volume = 25 mL = 25 / 1000 = 0.025 L
On putting these values in the molarity formula-
Molarity of H₂SO₄ = 0.005016 moles / 0.025 L
Molarity of H₂SO₄ = 0.20 M
Hence, the unknown concentration of H₂SO₄ is found to be 0.20 M.
To know more about titration, visit:
https://brainly.com/question/2728613
#SPJ4
Consider the following reaction
2H20 — 2H2 + 02
The rate of production of O2 is 3.9 x 10-¹ mols/s. How many seconds will it take to decompose 175g H20
Answers
Answer:
The chemical reaction 2H2+O2→2H2O 2 H 2 + O 2 → 2 H 2 O is classified as a synthesis reaction.
Explanation:
Which of the following is the correct Lewis structure for methane (CHA)?
O A. H-C-H
H
I-0-I
OB. H-H-C-H-H
0
H
O c. H:C:H
H
OD H-C-H

Answers
The correct lewis structure of methane(CH4)
C. H:C:H:H
How do atoms become cations and anions? Please use specific examples in your explanation.
Answers
Answer:
Cations are ions with a net positive charge.
Cation Examples:
Silver: Ag+
Hydronium: H3O+
Ammonium: NH4+
Anions are ions with a net negative charge.
Anion Examples:
Hydroxide anion: OH-
Oxide anion: O2-
Sulfate anion: SO42-
Explanation:
Cations and anions are both ions. The difference between a cation and an anion is the net electrical charge of the ion. Ions are atoms or molecules which have gained or lost one or more valence electrons, giving the ion a net positive or negative charge. If the chemical species has more protons than electrons, it carries a net positive charge. If there are more electrons than protons, the species has a negative charge. The number of neutrons determines the isotope of an element but does not affect the electrical charge.
A polar covalent bond occurs when one of the atoms in the bond provides both bonding electrons.a. Trueb. false
Answers
A polar covalent bond occurs when one of the atoms in the bond provides both bonding electrons. The statement is false.
A polar covalent bond occurs when two atoms share a pair of electrons unevenly, meaning that one atom has a greater electronegativity than the other atom.
This results in a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom, creating a dipole.
The situation described in the statement, where one atom provides both bonding electrons, refers to an ionic bond. In an ionic bond,
one atom transfers its electrons to another atom, creating a positively charged cation and a negatively charged anion. These oppositely charged ions are then attracted to each other, forming the ionic bond.
In summary, the statement is false because a polar covalent bond involves the unequal sharing of electrons between two atoms,
while the scenario described refers to an ionic bond where one atom provides both bonding electrons.
To know more about polar covalentrefer here
https://brainly.com/question/30261436#
#SPJ11
Liquid x is colourless. What physical property can be used to identify this liquid?
Answers
Answer:
Melting and Boiling points
Explanation:
The boiling point of a liquid is 100°C and the melting point is 0°C; this can help identify whether or not the liquid is pure or could potentially be water.
An aldehyde has the empirical formula CHO. What is the molecular formula if the molecular molar mass is 90.09 g/mol
Answers
The molecular formula of an aldehyde with the empirical formula CHO is C3H3O3.
What is the molecular formula of an aldehyde?First, we need to determine the empirical formula mass by adding the atomic masses of the atoms in the empirical formula:
C = 12.01 g/mol
H = 1.01 g/mol
O = 16.00 g/mol
Empirical formula mass = (1 x 12.01 g/mol) + (1 x 1.01 g/mol) + (1 x 16.00 g/mol) = 29.02 g/mol
Next, we can calculate the ratio of the molecular formula mass to the empirical formula mass:
ratio = molecular formula mass / empirical formula mass
We can calculate the molecular formula mass by dividing the given molar mass by the empirical formula mass:
ratio = 90.09 g/mol / 29.02 g/mol = 3.10
This means that the molecular formula has three times the number of atoms as the empirical formula. Therefore, the molecular formula of the aldehyde is C3H3O3.
Learn more about empirical formula
brainly.com/question/14044066
#SPJ11
Between Na and Na+ which has larger size
Answers
Answer:
Na+ is smaller than Na because, it has given away one electron because of which the electron shielding gets stronger due to more protons and less electrons. Whereas, Cl- is larger than Cl because it has gained an extra electron and so, the no.07/12/2010
Explanation:
NEED HELP NOW
Which item does not contain a noble gas?
neon sign
mp3 player
flash camera
helium balloon
Answers
Answer:
may be mp3 player......
Answer:
mp3 player
Explanation:
Neon sign contains Neon
Flash camera contains Xenon
Helium balloon contains Helium
Which of the following quantities are required for calculating density? Select all that required.
Volume
Area
Mass
Weight
Answers
Answer:
Mass and Volume
Explanation:
The formula for density is
\(\frac{Mass}{Volume}\)
Convert 0.30 m to mm.
Answers
Did the addition of salt change a physical or chemical property of water?
Answers
Answer:
Chemical
Explanation: By dissolving it is no longer salt by itself so it is a chemical change
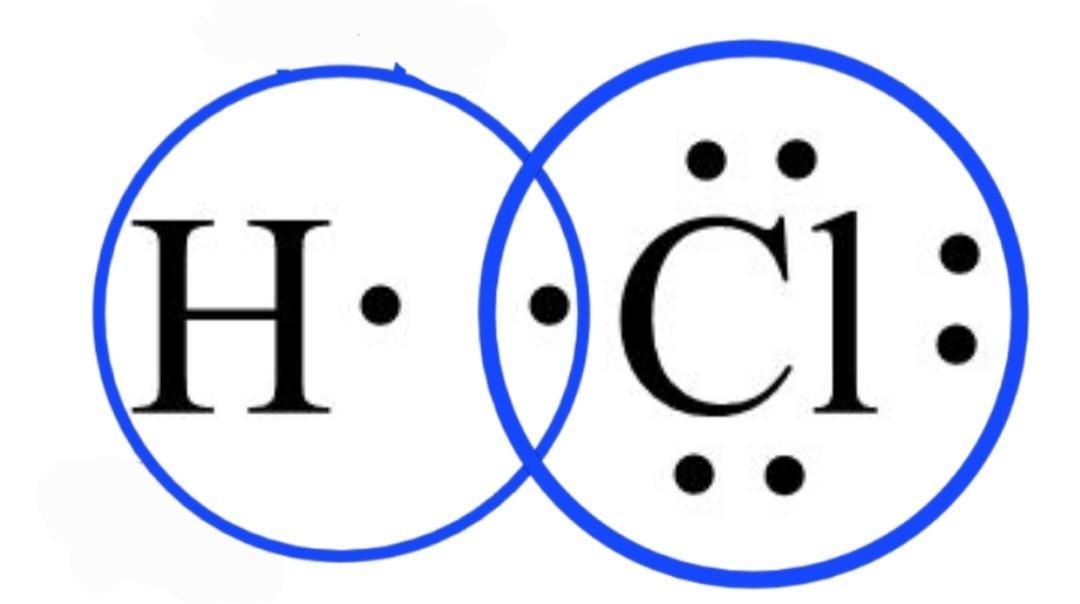
please answer fast!! very easy! correct answer will get 30 points!

Answers
The electron-dot formula of the hydrogen chloride HCl is shown in the attached diagram below.
What is the lewis electron dot diagram?A lewis electron dot structure can be used to express the number of bonds, the bonding atoms, and the lone pairs remaining in the atoms in the molecule.
Lines are represented between atoms that are bonded with each other and excess electrons or lone pairs are shown as dot pairs and are placed next to the atoms.
As the valence electrons in the chlorine atom are equal to 7 from the electronic configuration of the chlorine atom. First, the total number of valence electrons in the HCl molecule is 7 + 1 = 8.
A chlorine atom requires only one electron to complete its octet configuration. As the octet completes, the rest of the electrons of Cl are assigned as the lone pairs of the Cl atom. Therefore, the chlorine atom has 3 lone pairs of electrons on it and the hydrogen atom completes its duplet.
Learn more about the lewis dot diagram, here:
brainly.com/question/14091821
#SPJ1
What kind of reaction does this make?2 C₅H₅ + Fe ⟶ Fe(C₅H₅)₂A. Synthesis (S)B. Decompostion (D)C. Single Displacement (SD)D. Double Displacement (DD)E. Combustion (C)
Answers
The answer is option
The reaction:
\(2C_{5_{}}H_5+Fe\rightarrow Fe(C_5H_5)_2\)is a Synthesis reaction, because from 2 different substances it is produced
Calculate the P.N.E. for Ca(2+)
Answers
Answer: Atomic # for Ca = 20
protons for Ca = 20
electrons for Ca = 20
Atomic # for Ca(2+) = 20
protons for Ca(2+) = 20
electrons for Ca(2+) = 18
Explanation:
While working in a pharmaceutical laboratory, you need to prepare 1.50 L of a 2.20-M NaCl solution. What mass of NaCl would be required to prepare this solution
Answers
193 grams of NaCl are needed to make 1.50 L of a 2.20-M NaCl solution.
Calculation of the mass of NaCl required:
The only thing we need to know is that a solution's molarity informs us of the exact amount of moles of solute that are contained in 1 L of a solution.
In this situation, a NaCl (sodium chloride) solution with a 2.20-M concentration will have 2.20 moles of the solute (sodium chloride) in every 1 L of the solution.
The solution's molarity of 2.20 M requires that every 1 L of this solution include 2.20 moles of sodium chloride, which implies that every 1.50 L of this solution needs to contain
\(1.5 L solution .\frac{2.20 moles NaCl}{1L solution} = 3.30 moles NaCl\)
Utilize the compound's molar mass to translate the amount of NaCl (sodium chloride) in moles to grams.
\(3.30 moles NaCl . \frac{58.4 gm}{1 mole NaCl} = 192.7 gm\) ≈ 193 gm
Therefore it is concluded that the final answer is 193 gm.
Learn more about NaCl here:
https://brainly.com/question/18248731
#SPJ1
A fractionating column 2 1m in outside diameter is to be installed. The following specifications are available Shell length 45 m, Operating Pressure = 5 kg/cm², operating temperature 150 %. Skart height 4m, Tray spacing=0.5 m, Weir height=45 mm, Top disengagement space 1.0 m. Bottom separation space 1.5 m. Tray loading with liquid 110 kg/m², Tray support rings = 50mmx 50mmX10mm angles, Corrosion allowance 3 mm, Insulation Thickness 100 mm, Permissible stress for shell material 800 kg/cm², Welded joint efficiency 100%, Density of shell material- 7800 kg/m³. Density of insutation - 600 kg/m³, Overhead vapour line 300 mm outside diameter Weight of ladder= 35 kg/m, Weight of 300 mm O.D pipe 80 kg/m., Wind force acting over the vessel 135 Kg/cm2 Suggest a suitable Mechanical design for a fractionating column. Yield stress of the material is 1355 Kg/cm²
Answers
Based on the given specifications, a suitable mechanical design for a fractionating column can be suggested.
The design should consider factors such as the shell length, operating pressure and temperature, tray spacing, tray loading, insulation thickness, material properties, and external forces such as wind. Proper selection of materials, structural components, and design calculations should be conducted to ensure the column's safety and functionality.
To design the fractionating column, several factors need to be considered. The shell length of 45 m, along with the operating pressure of 5 kg/cm² and operating temperature, should determine the appropriate shell material and thickness. The permissible stress for the chosen shell material, along with the welded joint efficiency, should be taken into account in the design calculations.
The tray spacing, tray loading, weir height, and separation spaces will influence the tray design and support requirements. The column's insulation thickness, density of insulation material, and the required thermal insulation properties should be considered for efficient operation.
External forces such as wind force acting on the vessel and the weight of ladder and overhead vapor line should be analyzed to ensure structural integrity. The yield stress of the material should be checked against the design stresses to ensure the column can withstand the applied loads.
A comprehensive mechanical design should incorporate all these considerations, including material selection, structural components, calculations, and safety factors, to ensure a suitable and robust design for the fractionating column.
Learn more about fractionating column here: brainly.com/question/31839396
#SPJ11
Use thermodynamics and the concept of energy quality to explain why we can only burn a gallon of oil as fuel once
Answers
Thermodynamics is the branch of science that deals with energy conversion and the rules governing the movement of energy from one form to another. Energy quality refers to the level of usefulness of energy. It refers to the amount of useful energy that is available to perform a given task.
The first law of thermodynamics, also known as the Law of Conservation of Energy, states that energy can neither be created nor destroyed; it can only be converted from one form to another.
This means that the energy that we get from burning a gallon of oil cannot be destroyed but can be converted to other forms of energy like heat, electricity, and kinetic energy.
However, the second law of thermodynamics tells us that every energy conversion comes at the cost of some energy loss.
This means that every time we convert energy from one form to another, some of the energy gets lost in the form of heat. So, when we burn a gallon of oil, we cannot get all the energy that it contains in a useful form.
Instead, we get only a fraction of it as useful energy, and the rest is lost as heat.
Energy quality refers to the level of use of energy.
It refers to the amount of useful energy that is available to perform a given task.
Some forms of energy, like electricity, are very useful because they can be easily converted to other forms of energy.
Read more about Thermodynamics.
https://brainly.com/question/33422249
#SPJ11
How can a molecule like ozone have a molecular dipole when all of its bonds are non-polar?.
Answers
A molecule like ozone has a molecular dipole when all of its bonds are non-polar because they specifically have distinct electrical characteristics.
One oxygen atom is attached to the oxygen atoms on the ends, while two are bonded to the oxygen atom in the middle. The reason why the atoms are different is because of their surrounding environment. O3 is a polar molecule and this is because of the molecular geometry that is bent. In O3, a net dipole moment occurs as a result of the bonds' electric dipole moments not counterbalancing one another. That is why O3 (ozone) is polar in nature.
The lone pair in the directed orbital of the main oxygen atom is responsible for a large portion of the dipole moment of ozone. The other two oxygen atoms' valence electrons are located in orbitals with more directed characteristics. Any molecule that lacks a dipole moment is nonpolar. Polarity is a property of dipole-moment molecules.
Learn to know more about Ozone on
https://brainly.com/question/27139974
#SPJ4