Answers
Answer:
C. 2,2 dichloromethane
Related Questions
A 2.50 L sample of butane gas (C4H10), measured at 22.0
oC and 1.20 atm pressure, is combusted completely and
the carbon dioxide gas collected at the same pressure and
temperature. What volume of CO2 is produced?
Answers
Answer:
Same i guess hdjekfhkshfjfkr
The resultant volume of the carbon dioxide gas which is produced by the combustion of butane is 14.4L.
How do we calculate the moles of gas?Moles of any gas will be calculated by using the ideal gas equation as:
PV = nRT, where according to qustion for butane gas
P = pressure = 1.20 atm
V = volume = 2.50L
n = moles = ?
R = universal gas constant = 0.082 L.atm / K.mol
T = temperature = 22 degree celsius = 295.15 K
On putting values we get
n = (1.20)(2.50) / (0.082)(295.15) = 0.124 moles
Given chemical equation is:
C₄H₁₀ + 13/2O₂ → 4CO₂ + 5H₂O
From the stoichiometry of the reaction, it is clear that,
1 mole of C₄H₁₀ = produces 4 moles of CO₂
0.124 mole of C₄H₁₀ = produces 4×0.124=0.496 moles of CO₂
Now we calculate the volume for the carbon dioxide gas at the given pressure and temperature as:
V = (0.496)(0.082)(295.15) / (1.20) = 14.4 L
Hence required volume of CO₂ is 14.4 L.
To know more about ideal gas equation, visit the below link:
https://brainly.com/question/24236411
#SPJ2
plants contain a little carbon-14, but why do humans also contain carbon-14?
Answers
The amount of carbon-14 present in humans is typically around 150 parts per trillion (ppt) or less.
Carbon-14 (14C) is a radioactive isotope of carbon. This isotope is naturally occurring in the environment and is present in small amounts in all living organisms, including plants and humans.
Plants contain a little carbon-14, but why do humans also contain carbon-14?
Humans also contain carbon-14 because they consume plants and animals that have also been exposed to carbon-14. Carbon-14 is created in the atmosphere when cosmic rays interact with nitrogen gas (N2) in the air, which produces carbon-14 and hydrogen (H) atoms.
These carbon-14 atoms combine with oxygen (O2) to form carbon dioxide (CO2), which is then taken up by plants during photosynthesis. As humans consume plants and animals, they take in carbon-14 as well.
Therefore, humans contain carbon-14 because they consume plants and animals that have also been exposed to carbon-14.
The amount of carbon-14 present in humans is typically around 150 parts per trillion (ppt) or less.
Learn more about carbon-14 from this link:
https://brainly.com/question/16462349
#SPJ11
What is the mass of 6.02 x 1023 particles of rubidium carbonate
Answers
Answer:
Explanation:
84.97
Which of the following statements is FALSE?a. AgCl is predicted to be more soluble in pure water than in 0.10 M HClb. A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°Cc. Ag2CO3 is predicted to be more soluble in pure water than in 0.10 M HCld. AgCl is predicted to be more soluble in 0.10 M HCN than in pure water (Kf of Ag(CN)2− = 3 x 1020)
Answers
The FALSE statement among the given options is (b) A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°C.
When AgCl dissolves in water, it reacts with water molecules to form H+ and OH- ions, which leads to an acidic solution. Therefore, a saturated aqueous solution of AgCl is predicted to exhibit an acidic pH, not a neutral pH.Option (a) is true because AgCl is more soluble in pure water than in 0.10 M HCl due to the common-ion effect. Option (c) is false because Ag2CO3 is more soluble in 0.10 M HCl than in pure water. Option (d) is true because the formation of the complex ion Ag(CN)2− increases the solubility of AgCl in the presence of excess CN-.
To learn more about AgCl:
https://brainly.com/question/17102479
#SPJ11
I really need to understand this
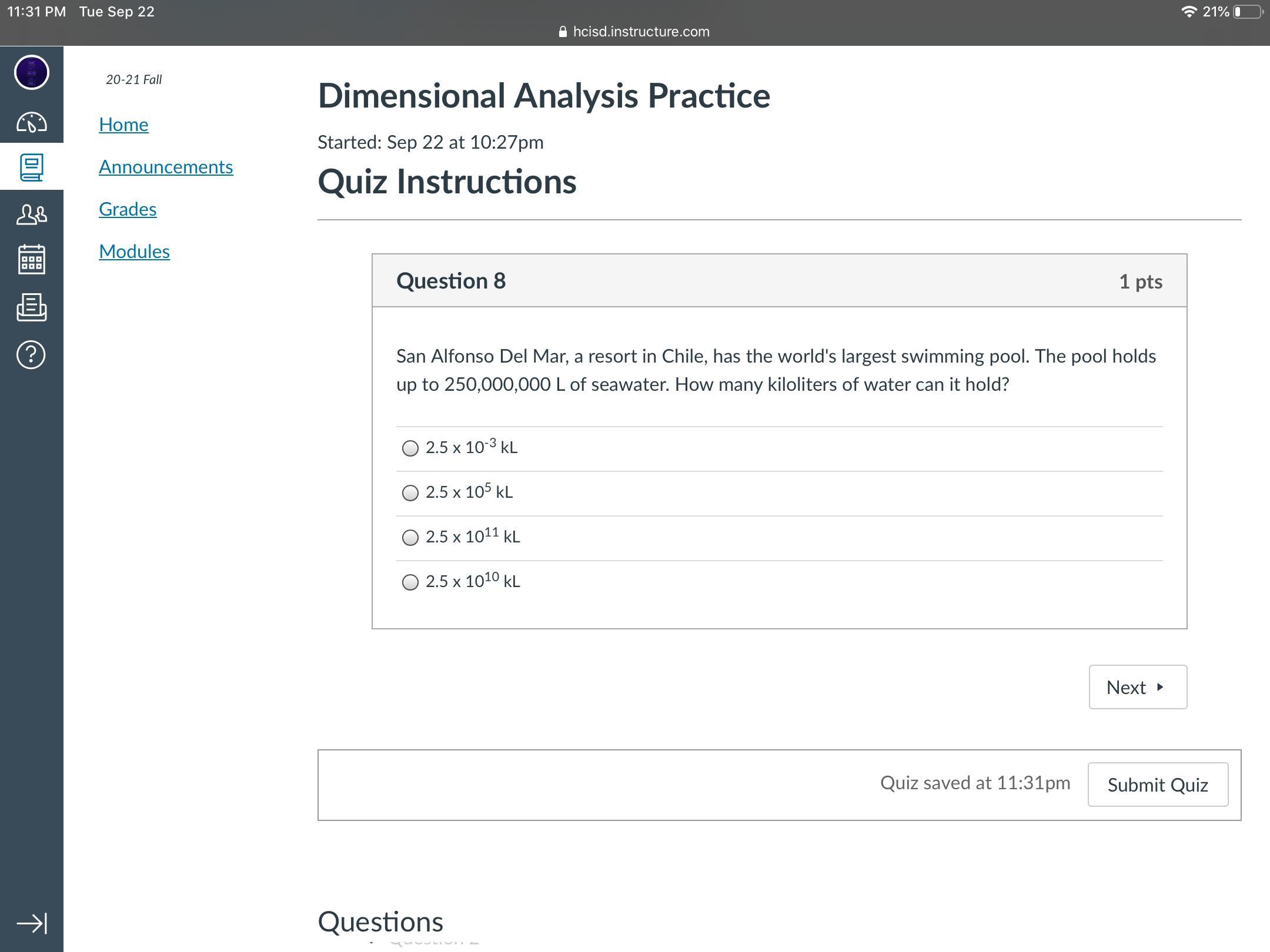
Answers
So the answer is 2.5 x 10^5 kL
I need yes or no I’m doing a element project and so is it good

Answers
explain why the first ionization energy is much lower than the second ionization energy for an atom of sodium.
Answers
The lower first ionization energy of sodium is due to the relatively weak attraction between the outermost electron and the nucleus, as well as the shielding effect provided by the inner electrons.
The ionization energy refers to the amount of energy required to remove an electron from an atom or ion in its gaseous state. In the case of sodium, the first ionization energy is significantly lower than the second ionization energy. This can be explained by understanding the electron configuration and the principles of electron shielding and effective nuclear charge.
Sodium has an atomic number of 11, meaning it has 11 protons in its nucleus and 11 electrons surrounding it. These electrons are arranged in energy levels or shells, with the first shell containing 2 electrons and the second shell containing 8 electrons. The outermost electron in sodium is in the third energy level.
The first ionization energy is the energy required to remove the outermost electron from the atom. In sodium, this electron is relatively far from the nucleus and experiences less attraction to the positively charged protons.
Additionally, the outer electron in sodium experiences significant electron shielding from the inner electrons, meaning that the inner electrons partially shield the outer electron from the full attractive force of the nucleus.
As a result, it is easier to remove the outermost electron in sodium, and hence, the first ionization energy is relatively low. Once the outermost electron is removed, sodium becomes a positively charged ion (Na+).
The second ionization energy refers to the energy required to remove an electron from the Na+ ion, which now has a stronger effective nuclear charge due to the reduced electron-electron repulsion and decreased shielding effect. Consequently, it is more difficult to remove an electron from the Na+ ion, leading to a higher second ionization energy compared to the first ionization energy.
For more such questions on ionization energy visit:
https://brainly.com/question/30831422
#SPJ8
What does the pacific tsunami warning system use to detect tsunamis ?
A. Radio signals In the air
B. Ripples in the water
C. Sensors on the ocean floor
D. Behavior of marine animals
Answers
Answer:
C
Explanation:
Their are pressure sensers in the water that will detect high pressure and set of scales that are currently detecting semic waves and trigger sierns.
Sodium fluoride inhibits the enolase reaction. Write out the reaction involved, giving structures and naming the compounds.
Answers
The enolase reaction is inhibited by sodium fluoride (NaF).
Enolase is an enzyme that catalyzes the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP) in the glycolysis pathway. The reaction involves the removal of a water molecule from 2-PG to form a double bond in the enol form, which is then converted to the keto form to produce PEP.
The reaction can be represented as follows:
2-Phosphoglycerate (2-PG) ⇌ Phosphoenolpyruvate (PEP)
The presence of sodium fluoride (NaF) inhibits this reaction. NaF is known to interact with metal ions, particularly magnesium ions (Mg2+), which are essential cofactors for enolase activity. NaF forms a complex with Mg2+ ions, reducing their availability for enolase, thus inhibiting its catalytic function.
Learn more about phosphoglycerate
brainly.com/question/31594691
#SPJ11
Persamaan berikut menunjukkan tindak balas antara asid sulfurik dan kalium hidroksida.Berapakah isi padu larutan kalium hidroksida 0.5 mol dm-3 yang boleh meneutralkan 50.0 cm3 asid
sulfurik 0.5 mol dm-3?
H2SO4 + 2KOH -> K2SO4 + 2H2O
A 25.0 cm3
B 50.0 cm3
C 100.0 cm3
D 400.0 cm3
Answers
Explanation:
2 KOH (aq) + H2SO4 (aq) →K2SO4 (aq) + 2 H2O (l)
what is the density of an object with a mass of 100 grams and a volume of 50 cm
Answers
Answer:
2
Explanation:
because 100÷50=2m÷v=d
Identify the acid associated with each conjugate base. I-SO4 2-Cl-OH -F-
Answers
The acid associated with each conjugate base is I-SO4 2= H2SO4 (sulfuric acid), ClO= HClO(hypochlorous acid), OH= H2O( water) and F- = HF (hydrofluoric acid).
For each conjugate base, the associated acid can be identified by adding a proton (H+) to the anion. The acid associated with each conjugate base is as follows:
I-SO4 2- : The conjugate base is derived from the acid H2SO4, which is sulfuric acid. The acid can donate two protons to form H+ ions and SO4 2- ions in aqueous solution.ClO- : The conjugate base is derived from the acid HClO, which is hypochlorous acid. The acid can donate a proton to form H+ and ClO- ions in aqueous solution.OH- : The conjugate base is derived from the acid H2O, which is water. The acid can donate a proton to form H+ and OH- ions in aqueous solution.F- : The conjugate base is derived from the acid HF, which is hydrofluoric acid. The acid can donate a proton to form H+ and F- ions in aqueous solution.Conjugate acid-base pairs are important in many chemical reactions, including acid-base reactions, redox reactions, and complex formation reactions. In acid-base reactions, for example, the acid donates a proton to the base to form its conjugate base and conjugate acid. The strength of the acid and base determines the position of the equilibrium in the reaction, with stronger acids and bases driving the reaction toward the formation of their weaker conjugate partners.
In summary, the concept of conjugate acids and bases is a fundamental aspect of acid-base chemistry and helps to explain the behavior of acids and bases in many different chemical reactions.
Learn more about conjugate base here:
https://brainly.com/question/30225100
#SPJ4
Which of the following values best classifies a bond between 2 atoms as
being covalent?

Answers
Answer:
C. An electronegativity difference of less than 1.7 between the atoms
Explanation:
I have no idea but that’s correct I just answered it on apex
An electronegativity difference of less than 1.7 between two atoms best classifies a covalent bond.
What is a covalent bond?
Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.These electron pairs are called as bonding pairs or shared pair of electrons.
Due to the sharing of valence electrons , the atoms are able to achieve a stable electronic configuration . Covalent bonding involves many types of interactions like σ bonding,π bonding ,metal-to-metal bonding ,etc.
Sigma bonds are the strongest covalent bonds while the pi bonds are weaker covalent bonds .Covalent bonds are affected by electronegativities of the atoms present in the molecules.Compounds having covalent bonds have lower melting points as compared to those with ionic bonds.
Learn more about covalent bond,here:
https://brainly.com/question/19382448
#SPJ2
in step 6 of the procedure, hcl was added to the reaction mixture to remove excess al(s). write a balanced chemical equation for the reaction between hcl (aq) and al(s). include physical states
Answers
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and aluminum (Al) is:
2HCl(aq) + 2Al(s) → 2AlCl3(aq) + H2(g)
In this reaction, the hydrochloric acid (HCl) reacts with the aluminum (Al) to produce aluminum chloride (AlCl3) and hydrogen gas (H2). The reaction is exothermic and occurs rapidly, releasing heat and hydrogen gas. The physical states of the reactants and products are indicated as follows:
HCl(aq) = Hydrochloric acid dissolved in water (aqueous solution)
Al(s) = Solid aluminum
AlCl3(aq) = Aluminum chloride dissolved in water (aqueous solution)
H2(g) = Gaseous hydrogen
To know more about referhydrochloric here
brainly.com/question/14006357#
#SPJ11
Together we help support an apparatus above the table surface
Answers
Answer:
The laboratory equipment which help to support an apparatus above the surface of the table are:
Ring Stand andClamps or ringsThey are used especially when there is need for heating by the Busen Burner.
Cheers!
10 POINTS!!! + BRAINLIEST A bird of mass 1.5 kg is flying at 8 m/s at a height of 50 m above the ground. What is its
total mechanical energy
Answers
Answer:12j
Explanation:
a liquid has a density of 1.05 g/ml. what is the volume, in liters, of 1.05 g of this liquid?
Answers
The density of the liquid is 1.05 g/ml. To find the volume of 1.05 g of the liquid, we can use the formula:
Density = Mass / Volume
Solving for Volume, we get:
Volume = Mass / Density
Substituting the given values, we get:
Volume = 1.05 g / 1.05 g/ml
Volume = 1 ml
To convert ml to liters, we divide by 1000:
Volume = 1 ml / 1000
Volume = 0.001 L
Therefore, the volume of 1.05 g of this liquid is 0.001 liters.
To find the volume of 1.05 g of a liquid with a density of 1.05 g/mL, you can use the formula:
Volume = Mass / Density
Given:
Mass = 1.05 g
Density = 1.05 g/mL
Now, plug the values into the formula:
Volume = 1.05 g / 1.05 g/mL = 1 mL
Since 1 L equals 1000 mL, you'll need to convert the volume from mL to L:
Volume = 1 mL * (1 L / 1000 mL) = 0.001 L
So, the volume of 1.05 g of this liquid is 0.001 liters.
To know more about density. please visit.....
brainly.com/question/27028246
#SPJ11
1.33 dm3 of water at 70°C are saturated by 2.25
moles of lead(II) trioxonitrate(V), Pb(NO3)2, and
1.33 dm3 of water at 18°C are saturated by 0.53
mole of the same salt\If 4.50 dm3 of the saturated
solution are cooled from 70°C to 18°C, calculate
the amount of solute that will be deposited in
(a) moles,
(Pb = 207, N = 14,0 = 16)
(b) grams.
Answers
Given that 4.50 dm³ of Pb(NO₃)₂ is cooled from 70 °C to 18 °C, the
amount amount of solute that will be deposited is 1,927.413 grams.
How can the amount of solute deposited be found?
The volume of water 1.33 dm³ of water 70 °C.
The number of moles of Pb(NO₃)₂ that saturates 1.33 dm³ of water at 70 °C = 2.25 moles
At 18 °C, the number of moles of Pb(NO₃)₂ that saturates 1.33 dm³ of water = 0.53 moles
Therefore;
Number of moles of Pb(NO₃)₂ in 4.50 dm³ at 70 °C is therefore;
1.33 dm³ contains 2.25 moles.
\(Number \ of \ moles \ in \ 4.50 \ dm^3 = \dfrac{2.25}{1.33} \times 4.50 \approx \mathbf{7.613 \, moles}\)
Number of moles of Pb(NO₃)₂ in 4.50 dm³ at 70 °C ≈ 7.613 moles
Number of moles of Pb(NO₃)₂ in 4.50 dm³ at 18 °C is therefore;
1.33 dm³ contains 0.53 moles
\(Number \ of \ moles \ in \ 4.50 \ dm^3 = \dfrac{0.53}{1.33} \times 4.50 \approx \mathbf{1.79 \, moles}\)
Number of moles of Pb(NO₃)₂ in 4.50 dm³ at 18 °C ≈ 1.79 moles
The number of moles that precipitate out = The amount of solute deposited
Which gives;
Amount of solute deposited = 7.613 moles - 1.79 moles = 5.823 moles
The molar mass of Pb(NO₃)₂ = 207 g + 2 × (14 g + 3 × 16 g) = 331 g
The molar mass of Pb(NO₃)₂ = 331 g/mol
The amount of solute deposited = Number of moles × Molar mass
Which gives;
The amount of solute deposited = 5.823 moles × 331 g/mol = 1,927.413 g
Learn more about saturated solutions here:
https://brainly.com/question/2624685
Why do atoms decay? explain if possible it can be simple
Answers
Answer:
Explanation:
In a nutshell, atoms decay because they're unstable and radioactive. Ununoctium (or Oganesson) has an atomic number of 118. That means that there are 118 protons in the nucleus of one atom of Oganesson, and that isn't including the number of neutrons in the nucleus.
Answer:
Atoms radioactively decay when a lower-energy nuclear configuration exists to which they can transition.
Explanation:
The actual decay event of an individual atom happens randomly and is not the result of the atom getting old or changing through time.
What is a static charge
Answers
Answer:
When you look only at the “before” and “after” of a change, you are considering it as static change. In this perspective, you look at change as a one-dimensional shift. A situation or object was one way, and now it is different because some outside force acted on it to make it change.
Explanation:
Its the def
Which cloud characteristic allows a provider to limit or charge by the amount of bandwidth, processing power, storage space, or client connections available to customers
Answers
The cloud characteristic that allows a provider to limit or charge based on the amount of bandwidth, processing power, storage space, or client connections is known as "resource pooling."
In the context of cloud computing, resource refers to any computing element or capability that can be allocated, utilized, and managed within a cloud environment. Resources can include various components such as processing power, memory, storage space, network bandwidth, virtual machines, databases, and software applications. Cloud providers offer a pool of resources that can be shared among multiple customers, allowing for efficient utilization and scalability. By provisioning resources on-demand, users can dynamically allocate and scale their resource usage according to their needs, optimizing efficiency and cost-effectiveness within the cloud infrastructure.
Learn more about resource here;
https://brainly.com/question/26767004
#SPJ11
Raoul ate a meal of oats before seeing his doctor. Oats are made of mostly starch molecules. Raoul feels tired, so his doctor gave him a test and found that raoul’s cells contained glucose molecules, but they did not contain enough oxygen molecules. Does this explain why raoul feels tired?.
Answers
No, his digestive system is breaking down starch molecules, but his respiratory system may not be working properly.
Oat starch which is mainly stored in the oat endosperm is the most abundant carbohydrate component of oats, and its content varies between 40-50% depending on the variety and growing conditions. Sayer and White 2011. Starch after extraction of oat amylose content lipids was determined by Gudmundson and Eliasson.
Whole grain oats contain large amounts of valuable nutrients such as protein starch unsaturated fatty acids, and dietary fiber in soluble and insoluble fractions. Starch is composed of two glucose polymers amylopectin, and amylose which together form insoluble semi-crystalline starch granules. Both polymers are composed of conjugated glucan chains connected by branch points, but their structure and biosynthesis differ.
Learn more about Raoul feeling tired here:-https://brainly.com/question/28751479
#SPJ1
What are the two types of carbohydrates and food sources?.
Answers
Answer: simple and complex.
Explanation:These are also called simple sugars. They're found in refined sugars, like the white sugar you see in a sugar bowl. If you have a lollipop, you're eating simple carbs.
determine the percent ionization of a solution having a ph of 4.35 and an initial weak acid concentration of 0.00019.
Answers
The percent ionization of a solution having a pH of 4.35 and an initial weak acid concentration of 0.00019 is 0.00021%.
To calculate this, first calculate the [H3O+] concentration.
This can be done by taking 10 raised to the power of the pH value, which in this case is 10^-4.35 = 3.2x10^-5 M.
Then, calculate the ionization fraction (alpha) using the equation alpha = [H3O+]/[HA], where [HA] is the initial weak acid concentration. In this case, alpha = 3.2x10^-5/0.00019 = 0.00021.
Finally, convert the ionization fraction to percent ionization using the equation Percent Ionization = 100 * alpha.
Thus, the percent ionization of the given solution is 0.00021 * 100 = 0.021%.
To know more about ionization, refer here:
https://brainly.com/question/14225136#
SPJ11
Select all reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction.
Answers
To carry out the bromination of benzene via an electrophilic aromatic substitution reaction, the following reagents are necessary: Bromine Br2, Lewis acid catalyst (Iron Bromide), organic solvent (tetrachloride).
1. Bromine (Br2) as the electrophile
2. Lewis acid catalyst such as iron (III) bromide (FeBr3) or aluminum bromide (AlBr3) to activate the bromine and enhance the electrophilicity of the system.
3. An organic solvent such as carbon tetrachloride (CCl4) or chloroform (CHCl3) to dissolve the reactants and provide a medium for the reaction to occur.
Bromine (Br2): This provides the bromine atom for substitution on the benzene ring. A Lewis acid catalyst, such as Iron(III) bromide (FeBr3) or Aluminum bromide (AlBr3): This helps generate the electrophilic bromine species and activates the benzene ring for the substitution reaction.
With these reagents, you can perform the bromination of benzene successfully.
To learn more about benzene click here
brainly.com/question/14525517
#SPJ11
The reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction are bromine (Br2) and a Lewis acid catalyst such as iron (III) bromide (FeBr3) or aluminum bromide (AlBr3). Additionally, a solvent such as nitrobenzene or carbon tetrachloride may be used to facilitate the reaction.
1. Bromine (Br2): This is the halogen that will be introduced to the benzene ring during the reaction.
2. A Lewis acid catalyst, typically either Aluminum Bromide (AlBr3) or Iron(III) Bromide (FeBr3): This catalyst is required to generate the electrophilic bromine species that will react with the benzene ring.
Your answer: The reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction are Bromine (Br2) and a Lewis acid catalyst, such as Aluminum Bromide (AlBr3) or Iron(III) Bromide (FeBr3).
To know more about electrophilic aromatic substitution reaction :
https://brainly.com/question/30761476
#SPJ11
OSSIBLES2
Mulchem with what would happen we have
Langs
Besi
III
Sko
Het
* Vedhere very little proteson against uyiri, Senal amage, ara pathogens the viruses are busers
35 Wd be able to be one to our body and remove carbon diote on our biker
45 Wd be able to gicky react to save ourselves from daneous stations
3 Wed he wable to crostate and deliver orygen ad carbon dioxide throughout our body
1
2
2
4
5
Next >
Answers
What makes metals, in particular, good conductors of electricity?
A.
the ability of electrons to flow throughout the metal
B.
the absence of charged particles
C.
the high temperatures required to break metallic bonds
D.
the presence of positive and negative ions
Answers
Answer:
the answer is A(the ability of electrons to flow through out the metal)
Define chemical reaction
Answers
Answer:
a process that involves rearrangement of the molecular or ionic structure of a substance, as distinct from a change in physical form or a nuclear reaction.
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
hope this helps ^^
Information on caffeine molecules with 3 uses and 3 properties
Answers
Answer:
it's uses
1)To improve mental alertness
2) For treating migraine headaches.
3) It increases the blood adrenaline levels
it's properties
1) It is white, odorless and hygroscopic crystalline solid
2) It tastes bitter
3) It is soluble on water
Don't forget to make me brainlist plz
Separate this redox reaction into its component half-reactions.
Cl2+2K→2KCl
Answers
The oxidation half-reaction is Cl_{2} → 2Cl- + 2e- and the reduction half-reaction is 2K+ + 2e- → 2K. These two half-reactions can be combined to give the balanced redox reaction.
In this redox reaction, chlorine gas (Cl_{2}) is reacting with potassium (K) to form potassium chloride (KCl). To balance the reaction, we need to identify the oxidation and reduction half-reactions.
The oxidation half-reaction involves the loss of electrons, and in this case, chlorine is being oxidized. The equation for this half-reaction is:
Cl_{2} → 2Cl- + 2e-
Here, each chlorine molecule (Cl_{2}) is being reduced to two chloride ions (Cl-) by losing two electrons (2e-).
The reduction half-reaction involves the gain of electrons, and in this case, potassium is being reduced. The equation for this half-reaction is:
2K+ + 2e- → 2K
Here, each potassium ion (K+) is being reduced by gaining two electrons (2e-) to form a neutral potassium atom (K).
By adding these two half-reactions together, we get the balanced redox reaction:
Cl_{2} + 2K → 2KCl
In summary, the oxidation half-reaction is Cl_{2} → 2Cl- + 2e- and the reduction half-reaction is 2K+ + 2e- → 2K. These two half-reactions can be combined to give the balanced redox reaction.
learn more about redox reaction Refer: https://brainly.com/question/2671074
#SPJ11
complete question: Separate this redox reaction into its balanced component half‑reactions. Use the symbol e− for an electron. Cl_{2}+2K⟶2KCl
oxidation half-reaction:
reduction half-reaction:
